In a time when the pharmaceutical industry is quickly adopting analytics, innovative ideas are evolving much faster into treatments. The emphasis on Clinical Data Interchange Standards Consortium (CDISC) benchmarks by regulatory agencies underscores the industry's shift toward advanced statistical approaches. Adhering to these standards not only ensures data precision and quality but also streamlines the drug development process.
Join us for a webinar where Maria Oxenbøll, Safety Surveillance Specialist at Novo Nordisk, sheds light on the significance of improved data workflows and the role of advanced tools like JMP® Clinical in shaping the future of clinical drug development.
Webinar highlights:
- Novo Nordisk discussing the evolution and efficiency of its data workflows.
- The transformative impact of advanced statistical methods in the realm of clinical trials.
- An overview of the advantages offered by an intuitive and easy-to-use platform for clinical reviews.
Key takeaways:
- Insights into the significance of streamlined data workflows for in-depth assessment.
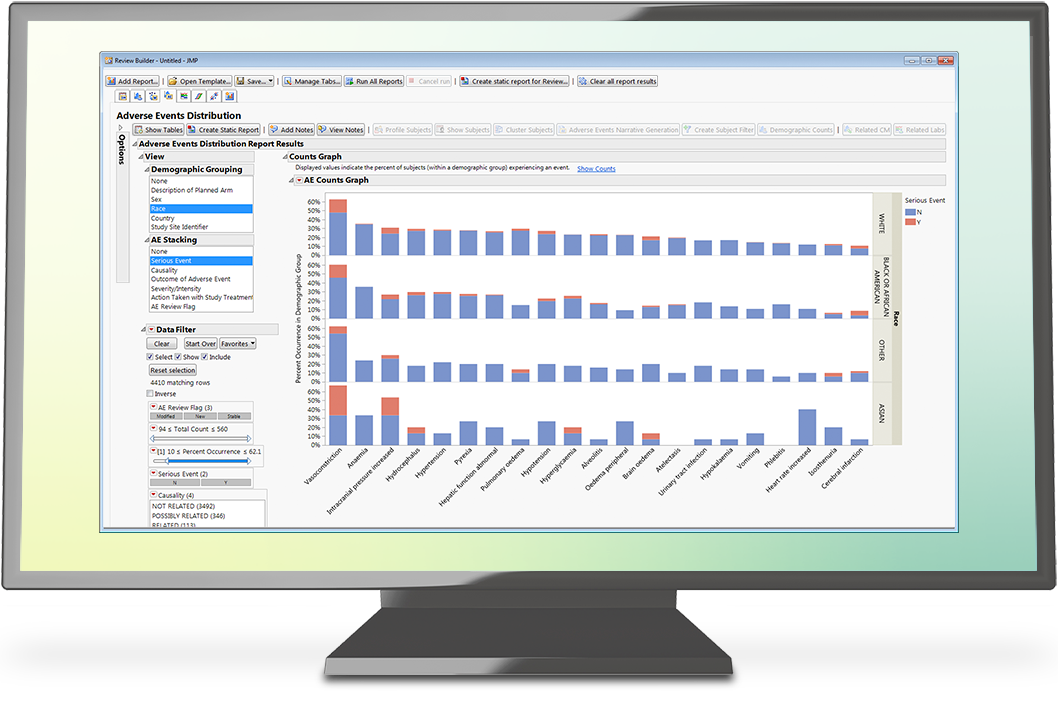
- An understanding of the pivotal role JMP Clinical plays in processing CDISC data sets quickly and easily to create on-the-fly interactive reports.
- The future trajectory of clinical drug development with the integration of advanced tools and methodologies.
- How to empower your inhouse analytical capabilities.
Who should attend?
This webinar is relevant for anyone who works with clinical data, including clinical data scientists, medical monitors, medical writers, data managers, clinical trial coordinators, and clinical research professionals.
Register now to be part of this enlightening session so you can have a better understanding of the transformative power of advanced data workflows in the pharmaceutical industry.