Many manufacturing processes involve hundreds or even thousands of process variables, each of which must be stable, or “in control.”
Moreover, the outputs of these processes – also often numerous – must all meet customer specifications.
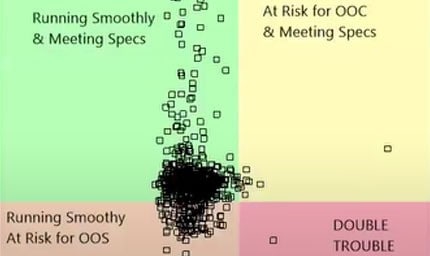
That’s a lot of opportunity for something to go wrong. When it does, you can’t afford to spin your wheels hunting for potential causes through trial and error.
In this 20-minute webinar, Rob Lievense shows you how to quickly focus your limited time and resources on the actions most likely to lead to meaningful process improvement. Through numerous examples and a case study, you will learn how to:
- Evaluate stability trends with process screening techniques.
- Run capability studies for many variables simultaneously.
- Identify the inputs with the highest potential of affecting a critical output.