Disease Response Swimmer Plot
This process creates a disease response swimmer plot and tables around either the best or last recorded response rates and calculated objective response rate for solid tumor oncology clinical trials, representing RECIST evaluation from the RS domain. The output includes a swimmer plot for subjects who have responded favorably to treatment for solid lesion trials per RECIST criteria using CDISC recommended evaluation responses of Complete Response (CR), and Partial Response (PR). Optionally, subjects can be annotated with responses of Stable Disease (SD) and/or Progressive Disease (PD) as well. The results of these assessments are summarized based on selecting either the Best response per subject, based on preferred order: CR, PR, SD, PD for controlled terms, respectively, or Last recorded response. Using this summarized response, subject counts and percentages of response rates are displayed in a summary table split by Treatment Variable based on report selection. The objective response rate (the sum and percentage of subjects who had CR + PR assessment result) is also calculated and listed in the summary table.
Report Results Description
Running this report for a modified Nicardipine study using default settings with the exception that both the Include Stable Disease and Include Progressive Disease Response options were checked.
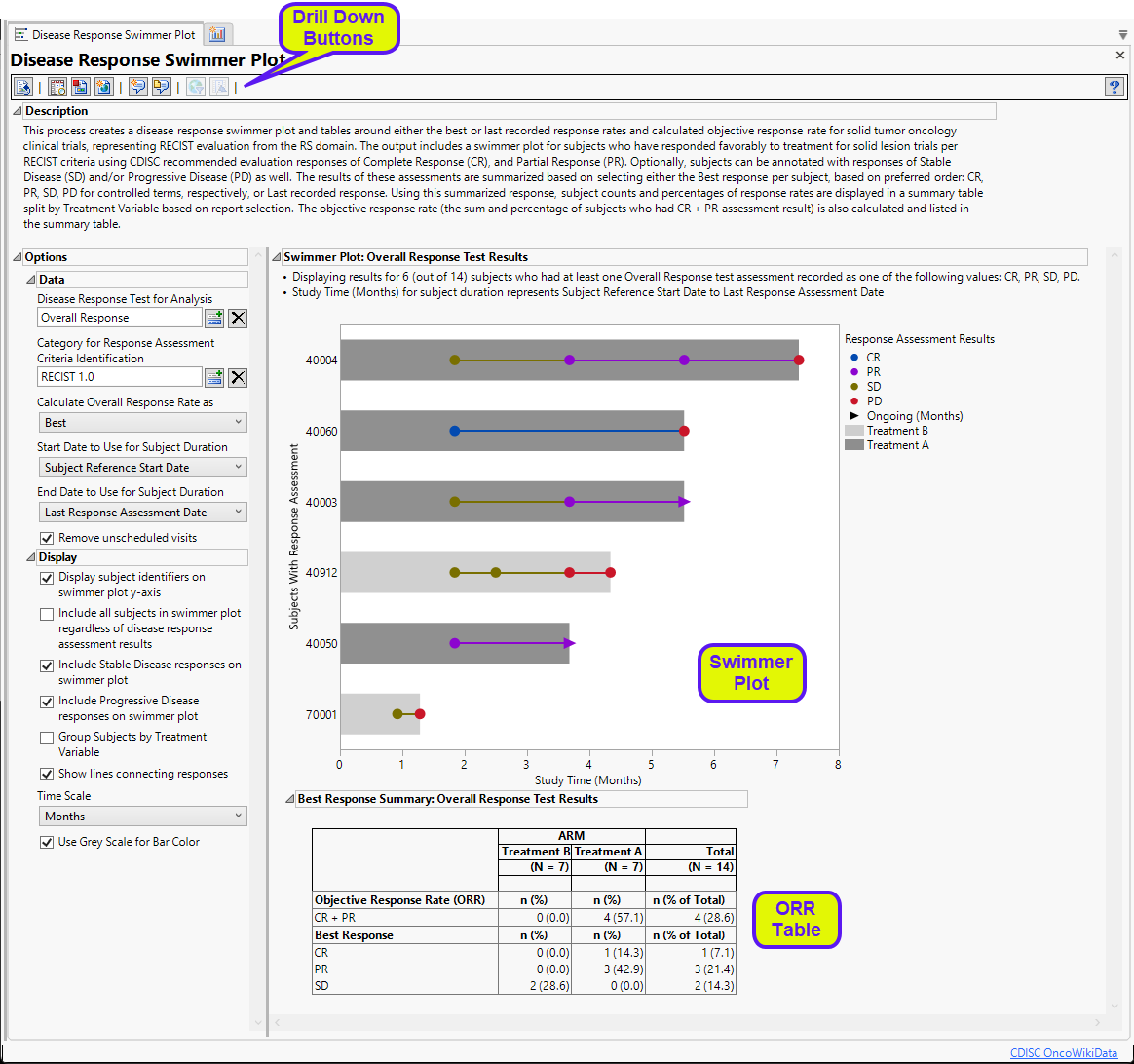
It contains the following elements:
| • | Summary text |
Summarizes the subjects plotted below.
| • | One Swimmer Plot |
The swimmer plots shows each subject’s response to treatment over the course of the study. Each bar represents one subject. Only those subjects who had at least one overall response assessment are plotted. The length of the bars correspond to the time each subject spent in the study and are colored by treatment. Reasons for leaving the study are not indicated. Response to the treatments are illustrated by the color of the dots, connecting lines, and arrows. These change color as treatment responses change. The dots indicate the time when the response was recorded. Arrows indicate ongoing response. This plot is a standard method for presenting these results.
Note: You can set your color preferences using theSet Value Order and Color in Studies utility.
| • | One Objective Response Rate (ORR) Summary table |
This table lists the numbers of the subjects showing complete response (CR) and partial response (PR), as well as those with stable disease (SD) for each treatment. Subjects showing progressive disease (PD) as their best response would also be listed. The table also shows the overall response rate (CR + PR) for each treatment. Total subject counts are also displayed.
Options
Data
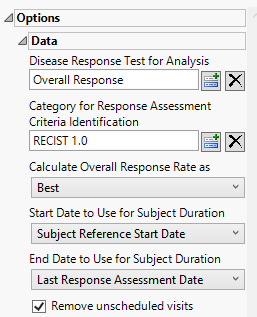
Disease Response Test for Analysis
Use the Disease Response Test for Analysis widget to select the relevant test code (RSTESTCD) whose results (RSSTRESC) contain values for tracking response assessment of Complete Response, Partial Response, Progressive Disease, or Stable Disease across study visits/time points. By default, if this option is left blank the report will attempt to find and use an RSTEST value of OVERALL RESPONSE (case insensitive).
Category for Response Assessment Criteria Identification
Use the Category for Response Assessment Criteria Identification widget to filter/select to the specific response assessment criteria value to use for showing response assessment in the resulting swimmer plot and table. If this field is left blank or your data does not contain the expected variable RSCAT with valid values of criteria identification, all records will be considered for the given selected test or algorithmically for any test named OVERALL RESPONSE.
Calculate Overall Response Rate as:
Use the Calculate Overall Response Rate as to select the option for which to calculate Response Rate Summaries displayed in the table for each subject. Choose Best to select a representative response that indicates the best response a patient had recorded at any time during response assessment using preferential logic of CR, PR, SD, PD order. Choose Last to take the last response assessment recorded for each subject. Note: If multiple responses are observed at the final visit, the best among them is reported.
Start Date to Use for Subject Duration / End Date to Use for Subject Duration
You must specify the start and end dates for collecting data to assess response of patients to the treatments. The Start Date to Use for Subject Durationand End Date to Use for Subject Duration widgets are used to select which variable date reference from the demography domain to use for calculating the response duration.
Remove unscheduled visits
Unscheduled visits can occur for a variety of reasons. By default, these are excluded from this analysis. However, by unchecking the Remove unscheduled visits box, you have the option of including them.
Display

Display subject identifiers on swimmer plot y-axis
By default, subject identifiers are not included on the swimmer plots. Click the Display subject identifiers on swimmer plot y-axis box to display the subject identifiers on the y-axes of the plots.
Include all subjects in swimmer plot regardless of disease response assessment results
You can include all subjects, regardless of disease response by checking the Include all subjects in swimmer plot regardless of disease response assessment results widget.
Include Stable Disease responses on swimmer plot
By default, stable disease responses are not included on the swimmer plots. You can include these responses by checking the Include Stable Disease responses on swimmer plot widget.
Include Progressive Disease responses on swimmer plot
By default, progressive disease responses are not included on the swimmer plots. You can include these responses by checking the Include Progressive Disease responses on swimmer plot widget.
Group Subjects by Treatment Variable
Swimmer plots are presented in order of longest to shortest time subjects have participated in the study, irrespective of treatment. The Group Subjects by Treatment Variable option enables you to group and color-code subjects by treatment.
Show lines connecting responses
Checking the Show lines connecting the responses widget connects the observations with lines.
Time Scale
By default, time is measured in months. However, you can change the Time Scale to plot time in either months or weeks. This option is useful for assessing report graphics for exceptionally long studies.
Use Grey Scale for Bar Color
You can opt to display the bars and response markers on the swimmer plot in grey scale by checking the Use Grey Scale for Bar Color widget.
General and Drill Down Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report:
| • | Click  to rerun the report using default settings. to rerun the report using default settings. |
| • | Click  to view the associated data tables. Refer to Show Tables/View Data for more information. to view the associated data tables. Refer to Show Tables/View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click  to open and view the Review Subject Filter. to open and view the Review Subject Filter. |
| • | Click  to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. |
Default Settings
Refer to Set Study Preferences for default Subject Level settings.
Methodology
The report scans the RS domain and summarizes the best or last disease response, depending on our selection, each subject exhibits to the treatment. Each result for complete and/or partial response is displayed and plotted over time. Further selection can display stable disease as well as progressive disease.