Standardized MedDRA Queries Incidence Screen
This report screens Standard MedDRA Query terms by performing a Cochran-Mantel-Haenszel exact test on all 2 x 2 tables constructed from SMQ incidence and treatment arm. Output includes one or more volcano plots of risk differences when using default settings. However, plots of relative risks or odds ratios can be generated depending on the selected option for the x-Axis for Volcano Plot.
Report Results Description
Running Standardized MedDRA Queries Incidence Screen for the Nicardipine study using MedDRA Version 16.0 files generates the report shown below.
Note: Your output might vary depending on which version of MedDRA you provide (in terms of the location of the *.asc files).
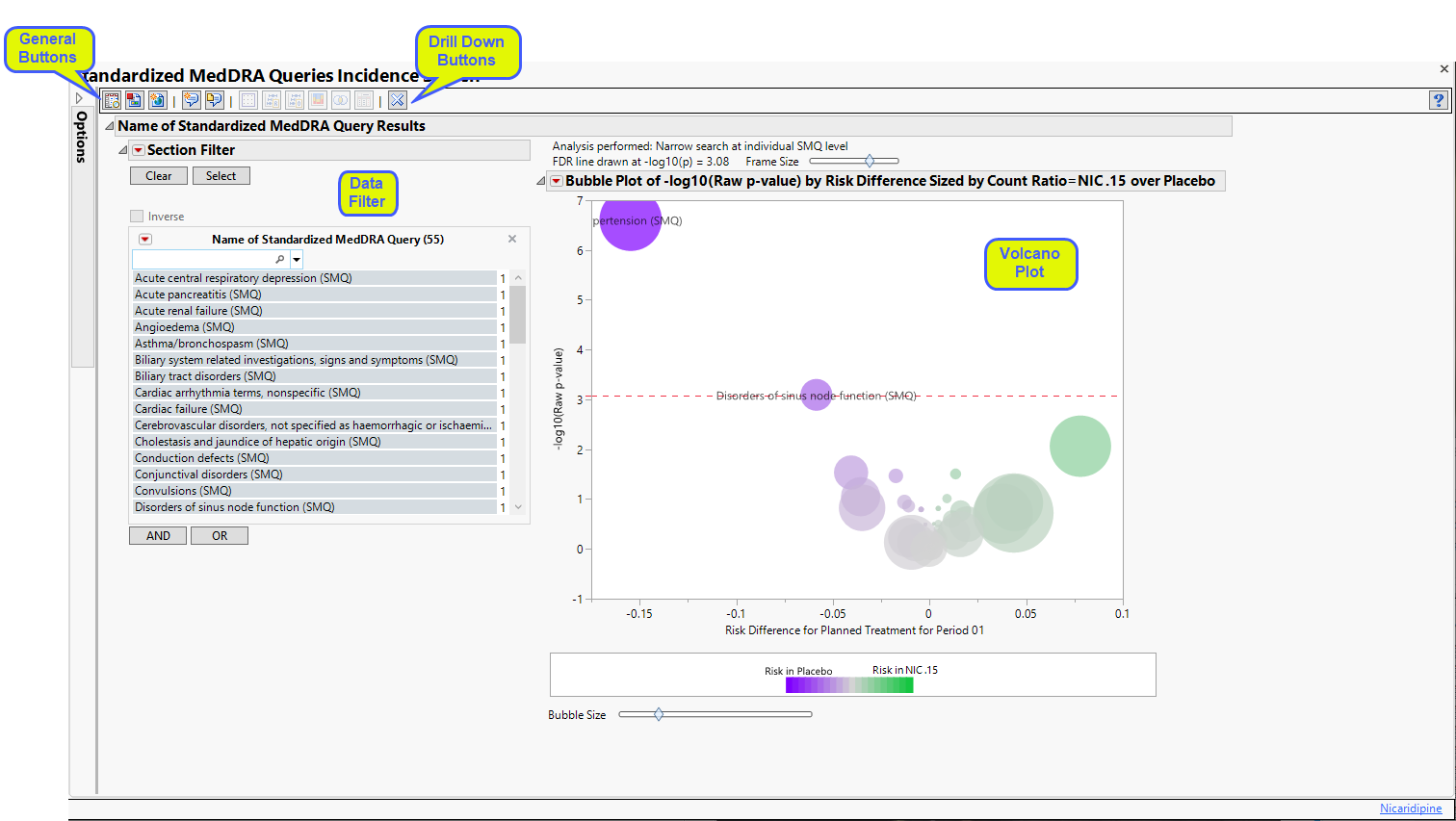
The Report contains the following elements:
Name of Standardized MedDRA Queries Results
Provides one or more Volcano Plots to summarize the incidence of SMQs between pairs of treatments. For the selected set of SMQs from the study dialog using Perform incidence analysis using the following SMQs:, one or more volcano plots are generated summarizing the difference in incidence between pairs of treatments.
Action Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report:
| • | Dot Plot: Click  to create a dot plot for selected terms or for all terms that fall under selected groups, with rates of adverse events by treatment, the relative risk, the risk difference, and the odds ratio presented. to create a dot plot for selected terms or for all terms that fall under selected groups, with rates of adverse events by treatment, the relative risk, the risk difference, and the odds ratio presented. |
| • | Relative Risk Plot: Click  to create a relative risk plot and table for selected terms or for all terms that fall under selected groups, with rates of adverse events by treatment, the log2(relative risk) and unadjusted 95% confidence intervals1 (Multiple Testing Method is not applied) presented. to create a relative risk plot and table for selected terms or for all terms that fall under selected groups, with rates of adverse events by treatment, the log2(relative risk) and unadjusted 95% confidence intervals1 (Multiple Testing Method is not applied) presented. |
| • | Odds Ratio Plot: Click  to create a plot that shows the ratio of the odds for exhibiting a selected event/intervention to the odds for those who do not exhibit the event/intervention and it can be used to estimate the relative risk when the probability of positive response is small. to create a plot that shows the ratio of the odds for exhibiting a selected event/intervention to the odds for those who do not exhibit the event/intervention and it can be used to estimate the relative risk when the probability of positive response is small. |
| • | Contingency Analysis: Click  to create the typical treatment by event contingency table for selected terms or for all terms that fall under selected groups. to create the typical treatment by event contingency table for selected terms or for all terms that fall under selected groups. |
| • | Tabulate: Click |
General
| • | Click  to view the associated data tables. Refer to Show Tables/View Data for more information. to view the associated data tables. Refer to Show Tables/View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click  to open and view the Review Subject Filter. to open and view the Review Subject Filter. |
| • | Click  to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. |
| • | Click the arrow to reopen the completed report dialog used to generate this output. |
| • | Click the gray border to the left of the Options tab to open a dynamic report navigator that lists all of the reports in the review. Refer to Report Navigator for more information. |
Report Options

Treatment or Comparison Variable:
The primary goal of clinical trials is to distinguish treatment effects when reporting and analyzing trial results. Treatments are defined by specific values in the treatment or comparison variables of the CDISC models. These variables are specified in this report using the Treatment or Comparison Variable to Use andTreatment or Comparison Variable options.
Distributions of the specified treatment or comparison variables are shown in the output.
Available variables include Planned, which is selected when the treatments patients received exactly match what was planned and Actual, which is selected when treatment deviates from what was planned.
You can also specify a variable other than the ARM or TRTxxP (planned treatment) or ACTARM or TRTxxA (actual treatment) from the CDISC models as a surrogate variable to serve as a comparator. Finally you can select None to plot the data without segregating it by a treatment variable.
See Treatment or Comparison Variable to Use, Treatment or Comparison Variable for more information.
Events to Consider
By default, all events are included in the analysis. However, you can opt to include only those considered serious. Selecting the Include serious adverse events only option restricts the analysis to those adverse events defined as Serious under FDA guidelines.
Analysis can consider all events or only those that emerge at specific times before, during, or after the trial period. For example, selecting On treatment events as the Event Type includes only those events that occur on or after the first dose of study drug and at or before the last dose of drug (+ the offset for end of dosing).
If you choose to Ignore available treatment emergent flags, the analysis includes all adverse events that occur on or after day 1 of the study.
By default, post-treatment monitoring begins after the patient receives the last treatment. However, you might want to specify an Offset for End of Dosing, increasing the time between the end of dosing and post-treatment monitoring for treatments having an extended half-life.
MedDRA Files
You must specify the location of the ASCI files downloaded from MedDRA.
See Location of MedDRA ASCII Files for more information.
Filtering the Data:
Filters enable you to restrict the analysis to a specific subset of subjects and/or adverse events, based on values within variables. You can also filter based on population flags (Safety is selected by default) within the study data.
If there is a supplemental domain (SUPPDS or SUPPMH) associated with your study, you can opt to merge the non-standard data contained therein into your data.
See Select the analysis population, Select saved subject filter2, Merge supplemental domain, Additional Filter to Include Adverse Events, and Additional Filter to Include Subjects
Performing the Test
By default, this report performs an incidence analysis of narrow SMQ terms individually without including any sub-SMQs. However, you can use the Perform incidence analysis using the following SMQs: option to alter the search. Search options include considering sub-SMQs in the analysis of the narrow SMQs, conducting your search at the broad SMQ level, with or without sub-SMQs, and restricting the analysis to SMQs that have an algorithm as part of the definition.
The Treatment Control Level is specified as either “Placebo” or “Pbo”, depending on the value found in your data, by default. However, if your control is defined differently you can use the text box to specify the control level is identified in your study.
You can also subdivide the subjects and run analyses for distinct groups by specifying one or more By Variables.
Stratification Variables segment the data into meaningful clusters and statistical tests such as the CMH exact test account for them. Such stratification is usually recommended when strata exist in your study, for example, by site.
Multiple Testing Adjustment Options
Multiple testing adjustments enable you to control both the proportion of all truly null hypothesis tests that are claimed to be significant (false positive rate) and the proportion of hypothesis tests claimed significant that are truly null (false discovery rate) for conclusions drawn across a collection of statistical tests.
The False Discovery Rate test is selected by default. You can use the Multiple Testing Method option to select alternative test protocols.
The Alpha option is used to specify the significance level by which to judge the validity of the summary statistics generated by this report. The meaning of alpha depends on the adjustment method that you select. Alpha can be set to any number between 0 and 1, but is most typically set at 0.001, 0.01, 0.05, or 0.10. The higher the alpha, the higher the error rate but also higher the power for detecting significant differences. You will need to decide on the best trade-off for your experiment.
Note that instead of performing multiple testing adjustments of the p-values, you can opt to simply specify a cutoff value for -log10(p-values) in order to select significant hypothesis tests. Using unadjusted p-values with a cutoff has the benefit of more expansive volcano plots, whereas adjusted p-values tend to squish points along the y-axis.Refer to -log10(p-Value) Cutoff for more information. Note: This option is available only when no multiple testing method is specified.
Results
Use the x-Axis for Volcano Plot option to specify the parameter to be plotted on the x-axis of your volcano plots. The Color Theme option enables you to specify the color scheme of the report graphics.