JMP Clinical Main Window
When you open JMP Clinical, the first window you see is the JMP Clinical Main window:
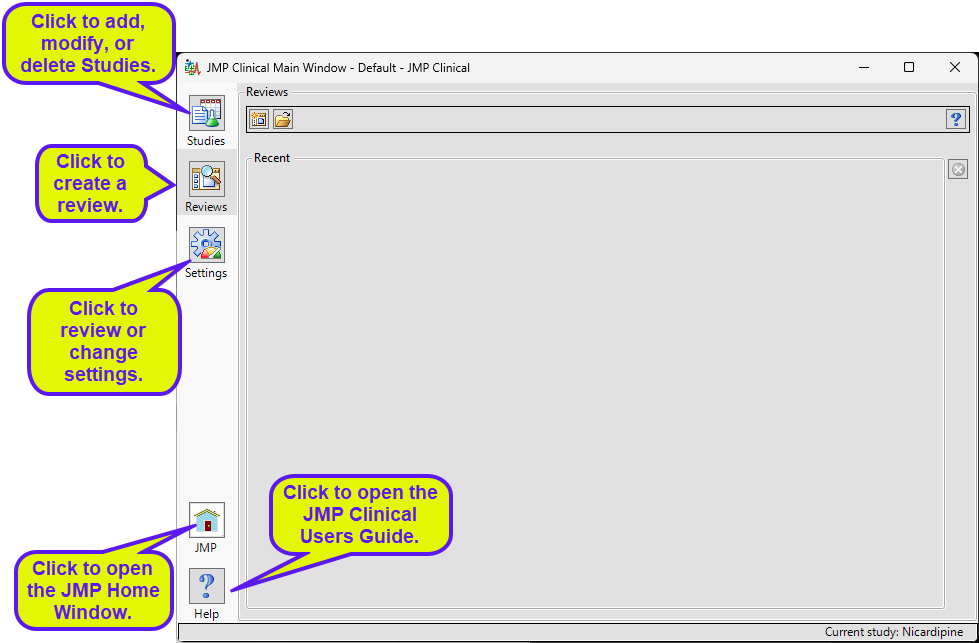
There are five buttons aligned on the left side of the JMP Clinical Main Window: Studies ( ), Reviews (
), Reviews ( ), Settings(
), Settings( ), JMP (
), JMP ( )and Help(
)and Help( ). The Reviews tab is selected by default.
). The Reviews tab is selected by default.
Click on the following links for more information:
The Studies Tab
Adding and Manipulating Studies
The Reviews Tab
The Settings Tab
A Note about User Roles
Before we can discuss specifics about each of the tabs on the JMP Clinical main window, we need to introduce the concept of the User Role in performing JMP Clinical analyses. JMP Clinical is designed as a multi-functional tool for setting up a study with the associated data, running a variety of reports and analyses, and reviewing the results.
By default a user can perform all of these functions and have access to all of the described functions and tabs. However, depending on the needs of your organization, these functions can be divided among different users, each carrying out a specific role. To facilitate these roles, JMP Clinical can be configured to provide individual users with the functional capabilities needed to carry out their assigned roles. As a result, when specific user roles are assigned, the JMP Clinical main window changes to show users only those functions specific to their assigned user role.
What follows here is a brief description of each of the JMP Clinical user roles, and their associated dashboards, that can be assigned by your system administrator1. Please note that because user roles can be combined (a review author can also be a settings editor, for example) the main window that you see might be somewhat different than shown here.
Study Manager
A user with this role can do all of the operations involved in managing studies within JMP Clinical: add, combine, and refresh metadata, rename or move folders, update a review with a new snapshot, update the study risk data set, and delete studies.
The “Study Manager” main window is shown below:
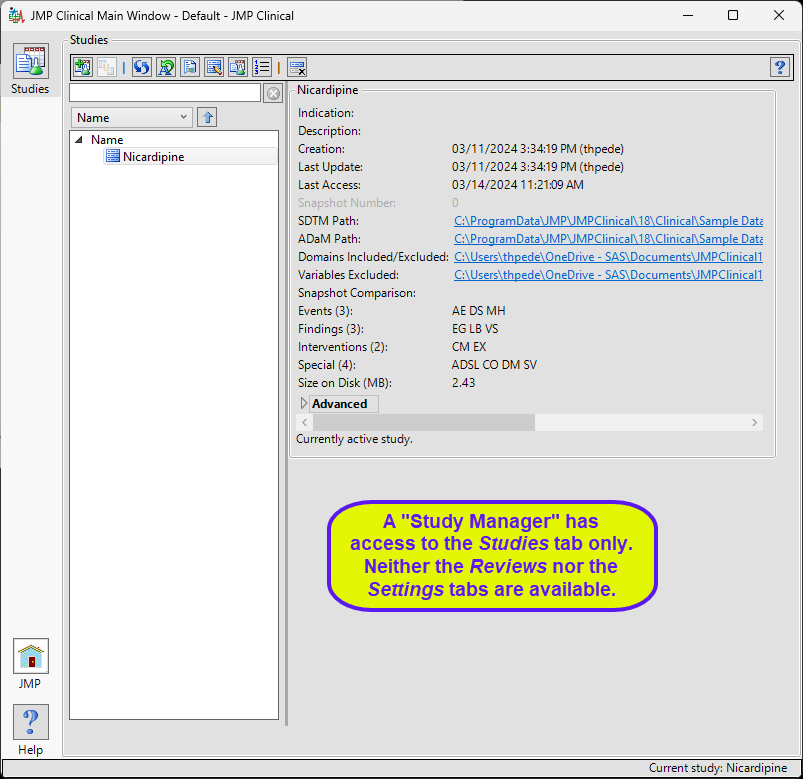
The study manager has full access to the Studies tab. Neither the Reviews tab nor the Settings tab, with their associated functions, are available to this user.
Study List Manager
A user with this role can select a study, view its snapshot history, explore the subjects in the study. They can also delete a study when the data files associated with it have been deleted.
The “Study List Manager” main window is shown below:
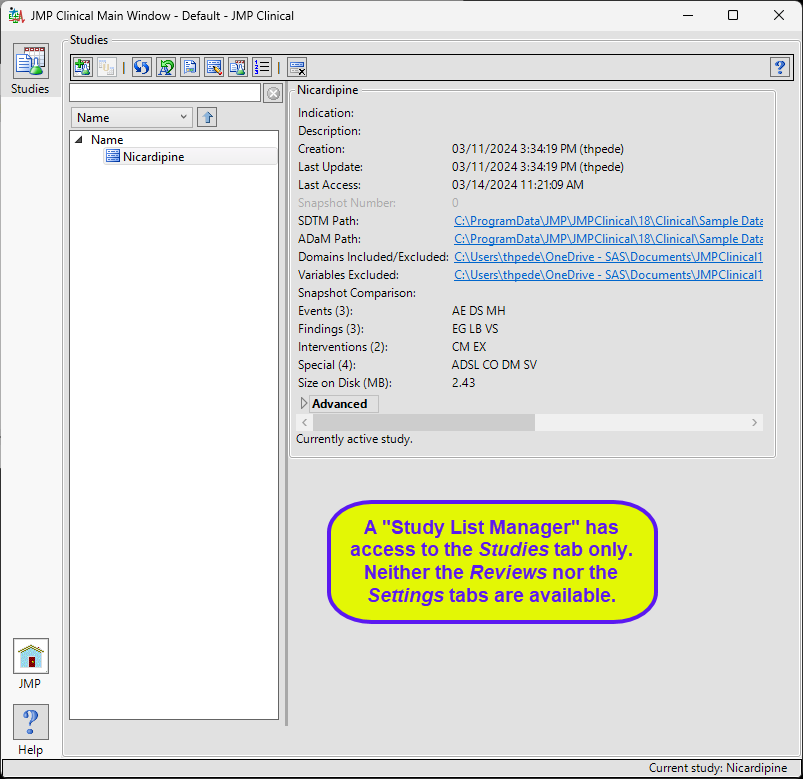
The study manager has limited access to the Studies tab. Neither the Reviews tab nor the Settings tab, with their associated functions, are available to this user.
Review Author
A user with this role can create/edit/save Review Templates, do ad hoc analyses, and create and save Reviews. They can also manage the Holiday and JMP Life Sciences / SAS Institute Event data set and Risk Threshold data sets used by the configuration. A Review Author implicitly has the Reviewer role as well.
The “Review Author” main window is shown below:
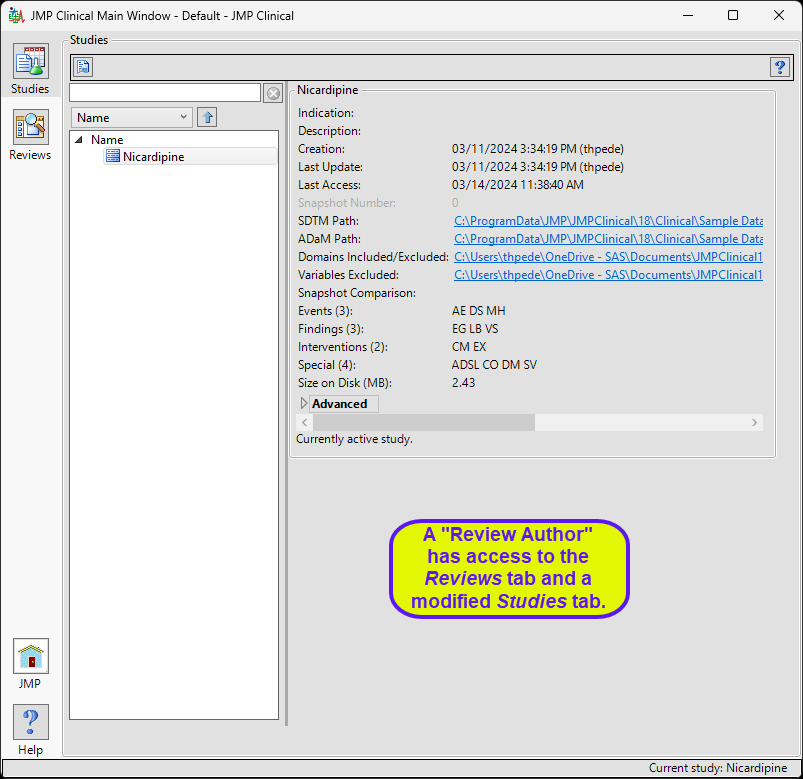
The review author has access to a modified Studies window where they can select a study, view its snapshot history, and explore the subjects in the study. Review authors can access all functions on the Reviews tabs and author new reviews and open existing reviews. The Settings tab is not available.
Configuration Manager
A user with this role can add and define JMP Clinical configurations. This role is assigned to all users by default.
Settings Editor
This role is normally found in conjunction with one or more of the other user roles. A user with this role has access to the Settings tab on the Main Clinical window. This enables them to select different configurations (if defined), change how they view Documentation and Help, and download and install a local copy of the documentation.
The “Settings Editor” main window is shown below:
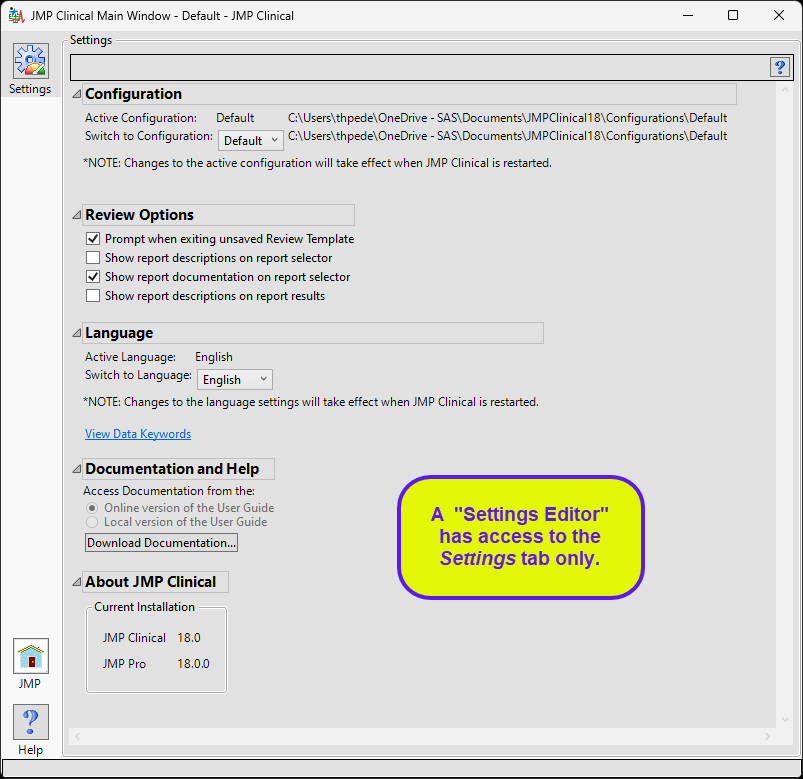
The settings editor has access to the Settings tab only.
Combined User Roles
JMP Clinical can be configured such that individual users have multiple roles. The Main Window surfaced to those users are permissive and show all the options available for each role.
Additional Information
Refer to Available Reports for links to information for each of the JMP Clinical reports.
Refer to Introduction for information about:
| • | System Requirements, Text Conventions, How is JMP Clinical affected by localization-specification?, Adverse Events Narratives, and The Example Data. |
| • | Frequently Asked Questions |
| • | System Operations and how JMP Clinical interacts with the clinical and nonclinical study data that is added to a JMP Clinical Study |
Refer to the Appendixes for a Glossary, Trouble Shooting guide, and other useful information.