Treatment Emergent Adverse Events Summary
This report creates tabular and graphical overviews of treatment emergent adverse events in the study by treatment arm.
Note: For information about how treatment emergent adverse events (TEAEs) are defined in JMP Clinical, please refer to How does JMP Clinical determine whether an Event Is a Treatment Emergent Adverse Event?.
Report Results Description
Running Treatment Emergent Adverse Events Summary for Nicardipine using default settings generates the Report shown below.
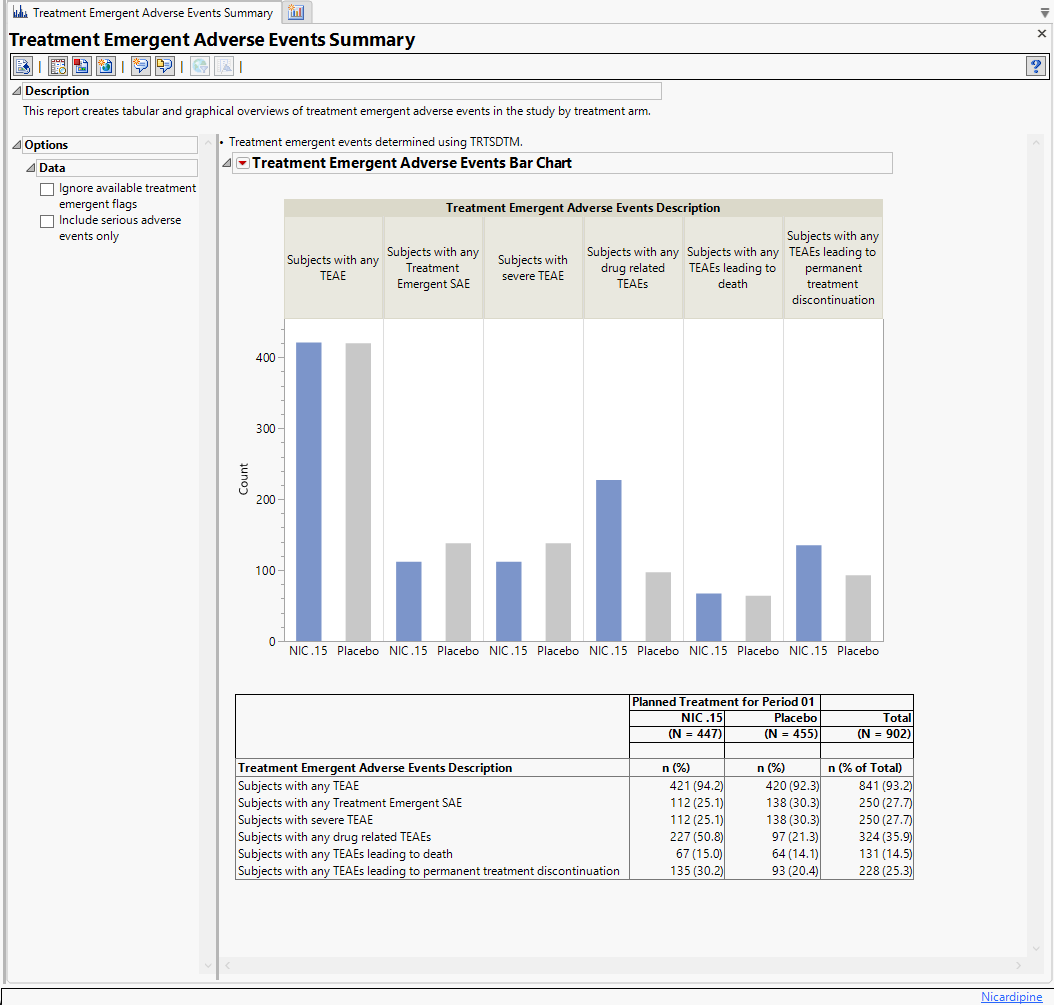
The following sections are generated by this report:
| • | Bar Chart: This section contains a Bar Chart summarizing the counts of various treatment emergent adverse events by treatment. |
| • | Summary Table: This section contains a table that summarizes the treatment emergent adverse events. Percentages of subjects experiencing an event by arm are also included in this summary. |
Options
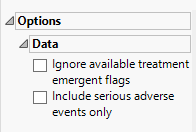
Data
Ignore available treatment emergent flags
If you choose to Ignore available treatment emergent flags, the analysis includes all adverse events that occur on or after day 1 of the study.
Include serious adverse events only
By default, all events are included in the analysis. However, you can opt to include only those considered serious. Selecting the Include serious adverse events only widget restricts the analysis to those adverse events defined as Serious under FDA guidelines.
Note: JMP Clinical preferentially uses AETOXGR over AESEV when assessing for severity. An AETOXGR value greater than or equal to 3 is used to flag an event as severe.
General and Drill Down Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report:
| • | Click  to rerun the report using default settings. to rerun the report using default settings. |
| • | Click  to view the associated data tables. Refer to Show Tables/View Data for more information. to view the associated data tables. Refer to Show Tables/View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click  to open and view the Review Subject Filter. to open and view the Review Subject Filter. |
| • | Click  to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. |
Default Settings
Refer to Set Study Preferences for default Subject Level settings.
Methodology
No testing is performed. Analysis is restricted to tabulating counts/percentages of subjects exhibiting treatment emergent adverse events.
Categories are displayed for subjects with any TEAE, subjects wtih any treatment emergent serious adverse event, subjects with severe TEAE, subjects with any TEAEs leading to permanent treatment discontinuation, subjects with any TEAEs leading to death, and subjects with any drug related TEAEs. Serious adverse events are determined using AESER equals Y. Severe events are determined using AETOXGR or AESEV with a value of 3 or greater for the former and a value of Severe for the latter. Permanent treatment discontinuation is determined using records where action taken with study drug (AEACN) is set to DRUG WITHDRAWN. TEAEs leading to death are determined using records where the outcome of the adverse event (AEOUT) is set to FATAL. Drug related TEAEs are determined using the causality variable (AEREL).
Causality
If a study has both ADaM and SDTM, then only the ADaM domain is loaded for the study. In this case, when ADAE and AE are both present, only ADAE is loaded. Causality is determined for ADaM and SDTM the same way. Also, observations with the values of N, NO, NOT RELATED, NOT, NONE, UNRELATED, UNLIKELY, UNLIKELY RELATED, REMOTE, or REMOTELY are all eliminated from causality consideration.