Crossover Analyses
Most large-scale clinical trials use a parallel experimental design in which randomly selected subjects are assigned to one of two or more treatment Arms. Once assigned to an Arm, each subject is given a single treatment, either the drug or drugs being tested, or the appropriate control (usually a placebo) for the duration of the study. Data are collected and subjected to between-patient analysis. Large sample sizes are usually required in these studies to account for the many sources of inter-subject variation, while still enabling accurate detection of any treatment effects.
An increasingly popular strategy for clinical trials, particularly for those involving stable, chronic conditions, involves the use of a crossover design. In this design, every subject is sequentially given all of the treatments in the study. Each treatment is administered for a defined period of time. Each subsequent treatment is preceded by a recovery, or washout, period where no treatments are administered, to allow subjects’ conditions to return to their normal states. Patients are randomized only with respect to the order in which the different treatments are administered. Because each subject serves as his or her own control, reducing the effects of between-patient variation, and because each subject can be used multiple times, crossover studies usually require far few subjects, although for longer times, than comparable parallel studies. These benefits can often outweigh the risks (patient drop-out, changing patient condition over time, carryover of one treatment to the next due to insufficient washout, or any secondary effects), associated with this design.
While JMP Clinical was initially designed for analysis of parallel studies, several reports in the JMP Clinical software now also support crossover analyses.
The following sections tell you how to use JMP Clinical for crossover analyses.
Using JMP Clinical for Crossover Analysis
Other JMP Clinical Crossover Analyses
Using JMP Clinical for Crossover Analysis
All study information must be recorded using an ADSL data set following the ADaM standard to support multiple treatment periods.
Required Variables
The variables in ADSL required for the system to support crossover include the following:
| • | Treatment Variables TRTxxP or TRTxxA: The Planned or Actual treatment for a given treatment period xx (The TRT01P, TRT02P, for example, would be the variables used to record the planned treatment for a two-period crossover analysis). |
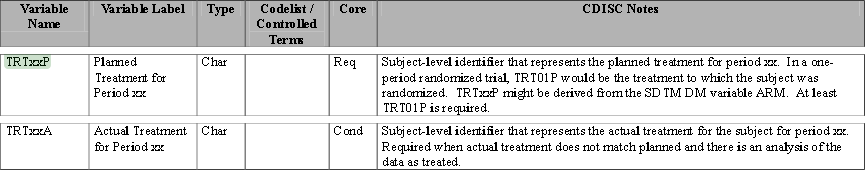
| • | Timing Variables (TRxxSDT or TRxxSDTM) and (TRxxEDT or TRxxEDTM): Dates or Date/Times in numeric format indicating the start and end dates respectively for each treatment period xx. |

Refer to the ADaM Structure for Occurrence Data Implementation Guide v1.1 for additional information.
JMP Clinical Reports Supporting Crossover Analysis
The software supports analysis and views for crossover trials in the following reports.
| • | Adverse Event Distribution |
| • | Adverse Event Incidence Screen |
| • | Adverse Event Severity ANOVA |
| • | Adverse Event Resolution Screen |
| • | Events Distribution |
| • | Events Incidence Screen |
| • | Findings Distribution |
| • | Findings ANOVA |
| • | Findings Time Trends |
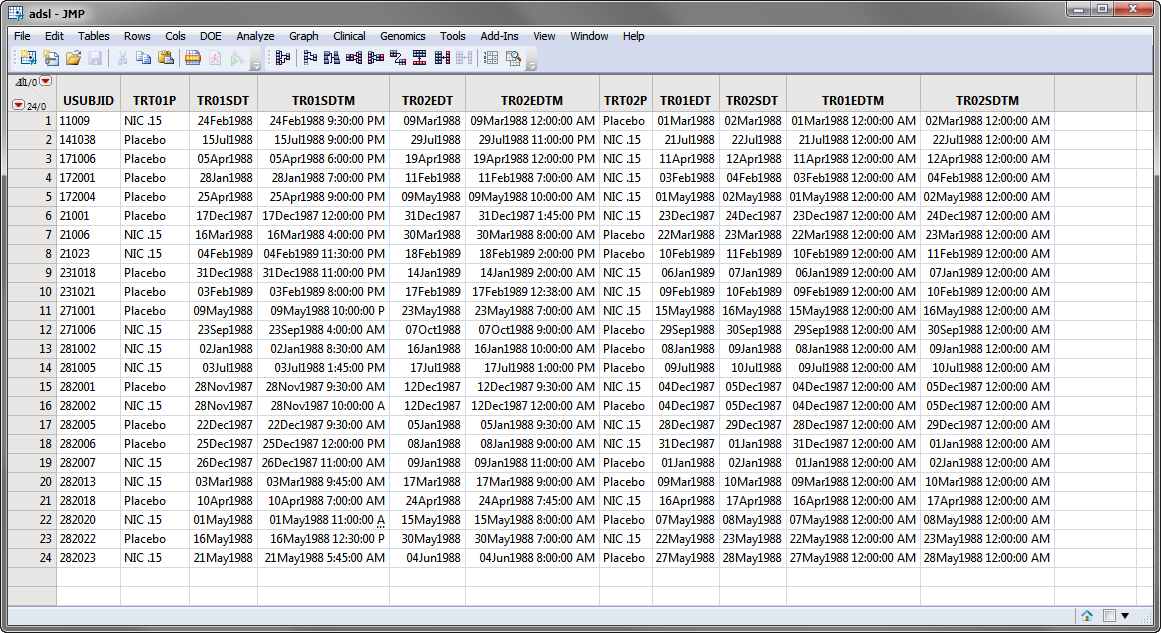
Currently crossover support is automatically detected and used if multiple TRTxxP or TRTxxA variables exist and the associated timing variables for the xx period exist and contain values. Those values might look like the values in the Nicardipine Crossover sample data set, shown below.
Note: For clarity, the majority of the adsl.sas7bdat columns in the screen shot below above have been hidden using the Cols > Hide/Unhide command.
Note: You can override crossover detection by specifying either Specified Below or None as the .
In the screen shot of adsl.sas7bdat, shown above, the SAS variable names are shown. During the report analysis, when adsl.sas7bdat is merged into the relevant analysis domain (for example, AE, LB, VS), the adsl treatment timing variables are compared to the timing variable in the domain and new variables: Treatment, Period, and Treatment (Period) are created. The values for these variables are assigned based on the value of the TRTxxP or TRTxxA and the value of the xx in the variable name when the start date of the domain record falls within the treatment period dates.
The SDTM data records for a subject can appear as shown in the portion of the VS domain (vs.sas7bdat (SAS names are being shown)) for the Nicardipine Crossover sample data set, shown below.
Note: For clarity, some of the vs.sas7bdat columns in the screen shot below above have been hidden using the Cols > Hide/Unhide command.
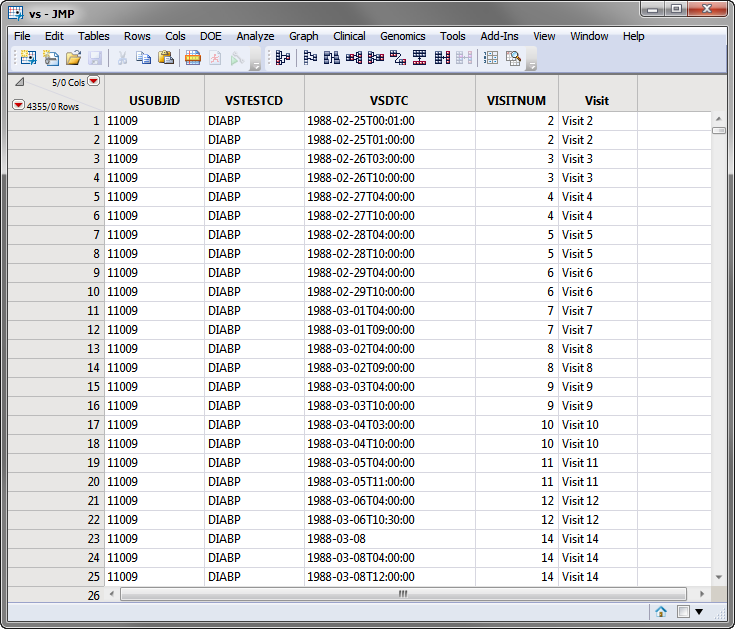
Based on comparison of the VSDTC date/time (note that SDTM follows the ISO 8601 date/time standard) with the ADSL timing date/time (numeric SAS date format), the subject records are assigned to the first treatment for Visits 1-6 and the second treatment period for Visits 7-14.
Similar analyses are done for events and intervention domains. In these domains, the timing comparisons for the record is based on the start date/time. For example, with the AE domain the AESDTC variable is used to assign the treatment period; indicating that the adverse event must START within the treatment period in order to be assigned that treatment value.
Report Results with Crossover Analysis
A sample Nicardipine Crossover analysis is now shipped with JMP Clinical. This can be loaded (using the button) from the Add Study from Folders dialog from the Clinical Starter Menu.
Analysis results and report options relevant to crossover analysis are shown below.
Options for visualizing crossover analysis are on the Findings Time Trend dialog include and . Both options are located on the Output tab of the report dialog. These options are discussed in more detail below.
Overlay visits when treatment crossover is detected
Check the check box to overlay visits when treatment crossover is detected for subjects.
Caution: You should check this box only if you want to overlay findings trends across the same time points (if detected) for treatments from different visits. Treatment crossover periods must coincide with Visits or unexpected results might occur. When this option is checked, any information about which treatment a subject was given at a visit is no longer discernible.
If time points are not detected within visits, this option is ignored.
Pool subjects in average time trend plots when treatment crossover is detected
Check the check box to pool subjects across treatment periods in the average time trend plots when a treatment crossover is detected. This results in the display of the average time trend plots for each unique treatment value across all treatment periods.
Note the following differences in output depending on whether the is checked:
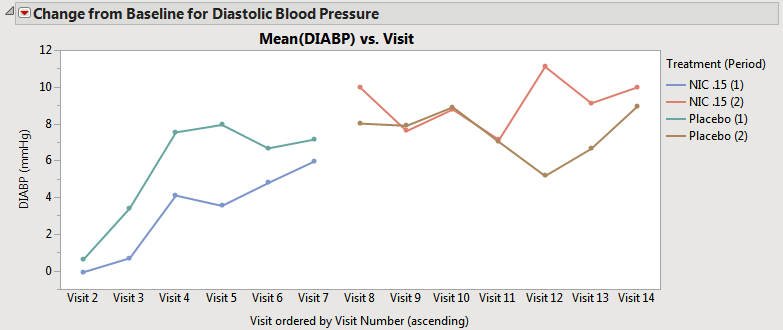
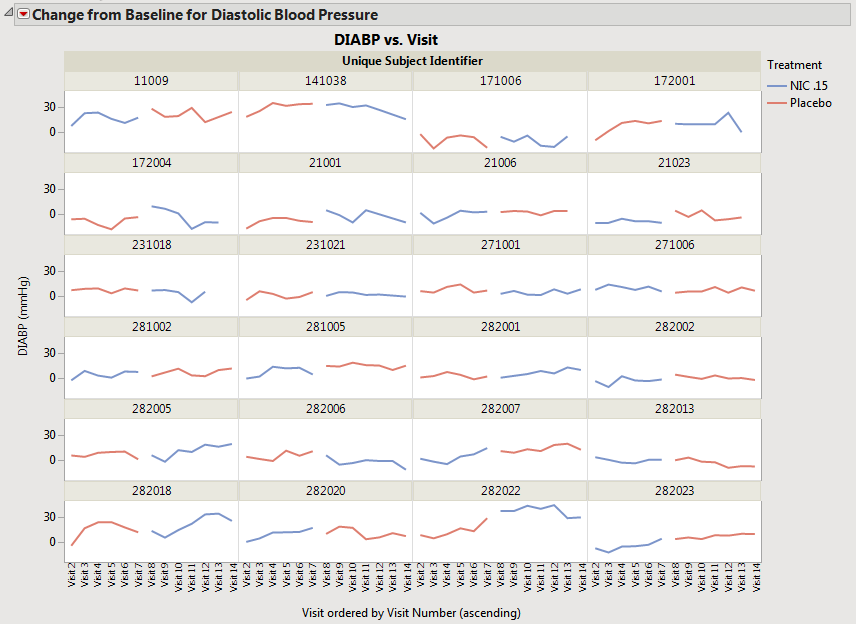
| • | When neither the box nor the box is checked, the average time trends view has one connected line representing the average for that treatment across all periods (assuming that the treatment value is coded with the same value across periods). Treatment values that changed across treatment periods still have separate lines at the moment of crossover (shown below). Note that in this case, this does not change the composition of the subjects used for calculating the means. |
| • | When the box is checked, but the box is not checked, the subjects that have the same value for treatment across different periods are pooled to calculate the average trend across the visit(s). For example, subjects assigned to Placebo on period 1 are pooled with subjects assigned to Placebo on period 2, and only one line is displayed for Placebo, as shown below. |

Note: When the box is not checked but the box is checked, two separate lines would be drawn for Placebo (1) and Placebo (2) (not shown).
| • | When Subject-level Time Trends Results are generated (occurs when the bow is unchecked), the results are same regardless of whether the option is checked or not. |
Note: Plots of the results obtained when the box is checked are not shown here because the example data does not contain time points.
When a crossover study is detected, the distribution results automatically categorizes adverse events by treatment period beginning with JMP Clinical version 5.0.
The results with the example Nicardipine crossover data are shown below.
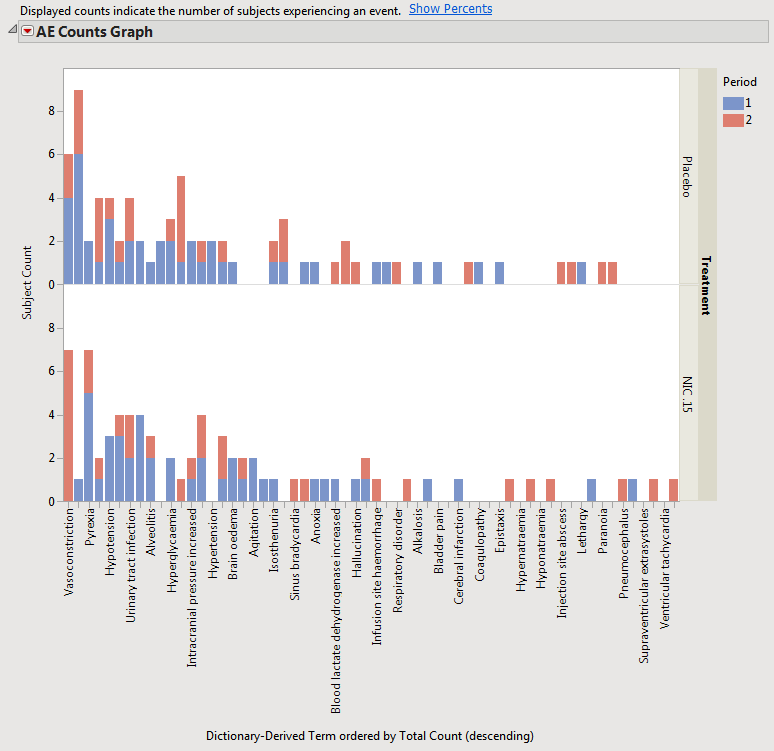
This view is similar for Interventions and other Events domains for distributions.
Other JMP Clinical Crossover Analyses
In the other relevant reports that support crossover, the results plots in the report are unchanged but treatment period is taken into account in the statistical model. For the incidence screen reports, when multiple periods are detected, the Unique Subject Identifier is automatically used as a STRATA variable to perform an incidence screen in the matched-pair framework analysis that adjusts for period and carryover effects. (Refer to Categorical Data Analysis Using SAS for more information.)1 In the Severity, Resolution, and ANOVA reports, treatment period is an additional fixed effect in the mixed model analysis (multiple repeated measures for subjects are already accounted for by the random effect component).