Combine Studies
This report creates a new study by combining two existing studies.
Running this report preserves all original data from studies to be combined, and creates new input data sets by appending rows from each corresponding parent data set. The USUBJID values of the new input data sets are unchanged. The new input data sets are then used to add the combined study to JMP Clinical. This option can combine studies with SAS data sets, SAS transport files, or both.
To combine two studies:
| 8 | Select the two studies to be combined and click 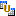 . . |
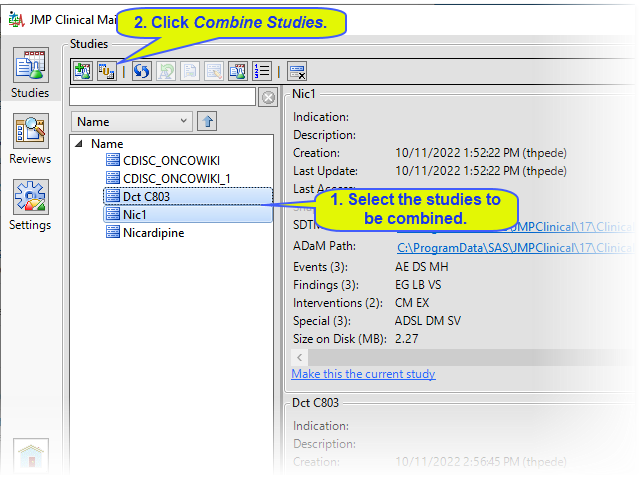
| 8 | Specify the names of the new study and the SDTM and ADaM folders and click . |
Links to specific documentation for each of the options are provided in the following table:
|
Options |
Information |
|||||||||||||||
|
||||||||||||||||
|
|
|
|||||||||||||||
|
|
|
|||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
Selecting this option enables future snapshot comparisons of study data collected and recorded at different intervals during the study period. |
||||||||||||||||
|
|
Note: In cases where there are variables that cannot be combined, this report generates a report listing those variables. |
| 8 | Specify the desired options and click . |
When the combination process is complete, the following Results window appears.
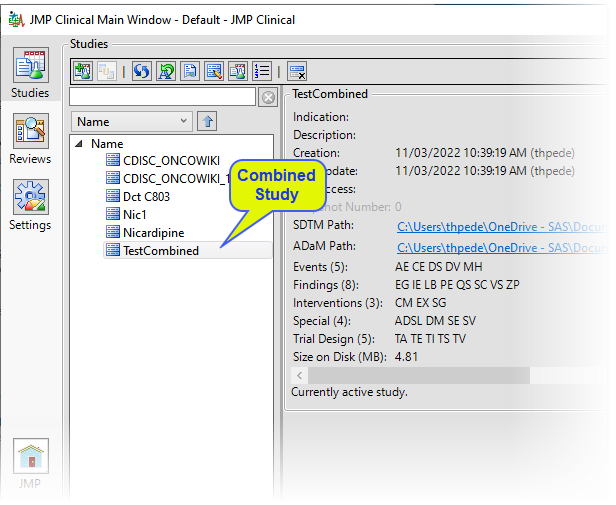
| 8 | Click to complete the process and add the combined study to the main window, as shown below. |