Add Study...
This study management action adds a study (see Studies) from either local folders or a SAS Drug Development (SDD) server that contain CDISC-formatted data. You must add a study before you can perform the analysis. This actioncan import JMP data tables (.jmp files), SAS data sets (.sas7bdat files), and SAS Transport Files (.xpt files) for you.
The following table summarizes the minimum requirements of new studies.
|
You must specify... |
...while abiding by these restrictions: |
||||||||||||||||||
|
|||||||||||||||||||
|
the location of either the SDTM Folder and/or the ADaM Folder |
|
The study and associated metadata are added to your database.
To Add a Study
| 8 | Click  to open the dialog: to open the dialog: |
| 8 | Specify the desired options and click . |
The following options are available. Links to specific documentation for each of the options are provided in the following table:
|
Options |
Information |
||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Selected SDTM Domains |
|
||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Selected ADaM Domains |
|
||||||||||||||||||||||||
|
Make this the current study |
|
Note: When adding a clinical study and assigning both ADAM and SDTM domains, the ADAM equivalent of each domain is added and the corresponding SDTM data set excluded. For example, if both ADAE and AE are present, then ADAE takes precedence and is included, whereas AE is not included.
Example: Adding the Nicardipine Study
JMP Clinical ships with the data from a Nicardipine study. The data files are located in the Sample Data in your Installation directory (typically C:\Program Data\JMP\JMPClinical\xx\Clinical\Sample Data\Nicardipine), where xx is the version of JMP Clinical. In this example, we add a clinical study we call Nic1.
| 8 | Click  to open the Add Study dialog. to open the Add Study dialog. |
| 8 | Specify the name of the study. |
| 8 | Specify the file paths to the SDTM and ADaM folders either by typing the path directly or clicking and navigating to the folders. |
The completed dialog appears as follows:
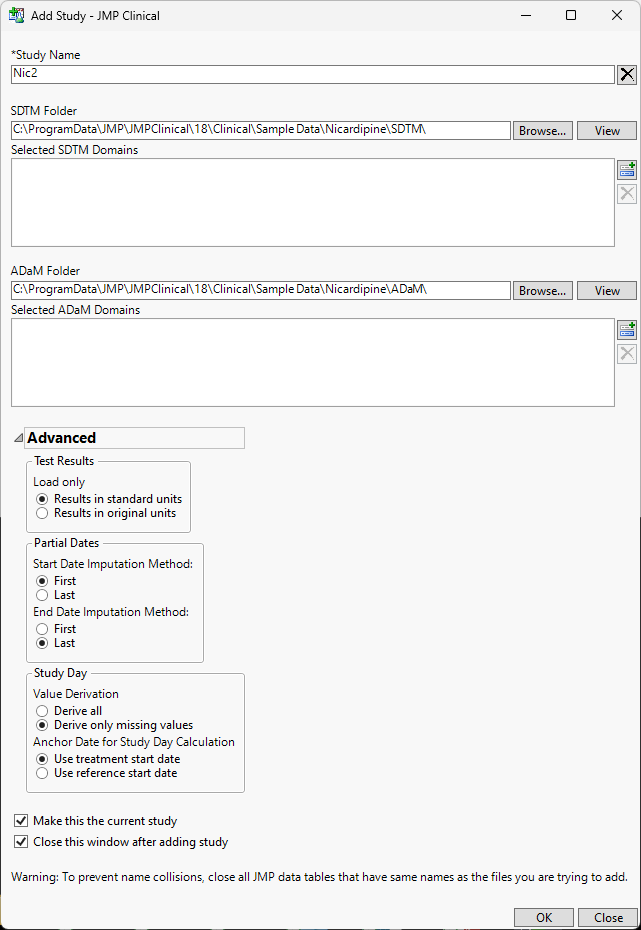
| 8 | Click to add the study. |
When a study has been successfully added, the new study appears in the Studies window.