Running
Standardized MedDRA Queries Distribution
for the
Nicardipine
study using
MedDRA
Version 16.0 files generates the
Report
shown below.
Note
: Your output might vary depending on which version of MedDRA you provide (in terms of the location of the *.
asc
files).
The
Report
contains the following elements:
Provides
Bar Chart
s to summarize the frequency or percentage of major classes of
SMQs
by treatment.
Bar charts
are provided to summarize the major classes of
SMQ
: a) Narrow Search at the Individual Term Level (open by default), b) Broad Search at the Individual Term Level, c) Algorithm Search at the Individual Term Level, d) Narrow Search at the Cumulative Term Level, or e) Broad Search at the Cumulative Term Level. The bars display the frequency or percentage by treatment of subjects that meet the criteria for each SMQ.
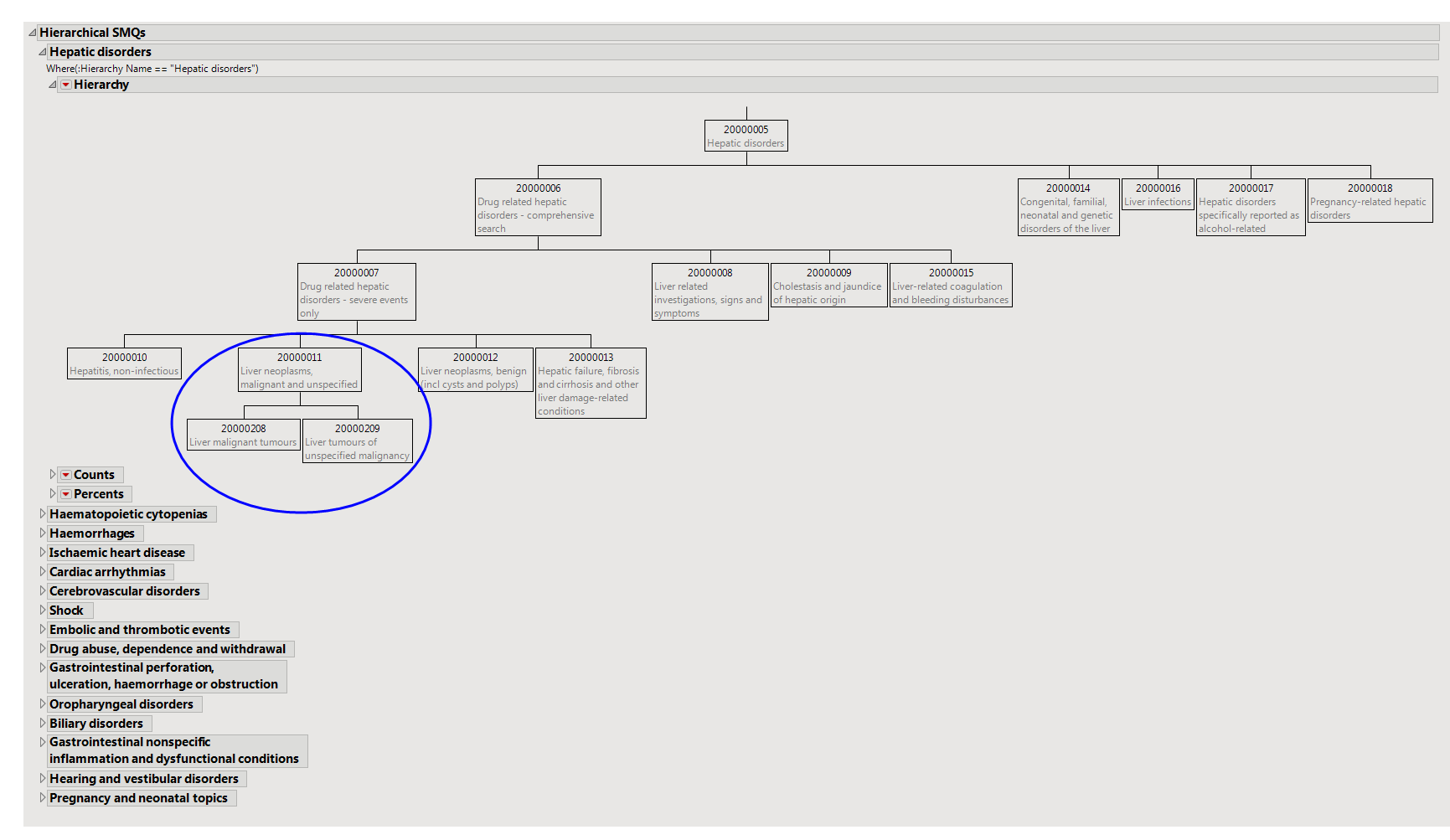
For non-hierarchical SMQs, the results of individual or cumulative analyses are identical. For hierarchical SMQs, cumulative analyses include SMQs from all sub-SMQs. For example, view the Hepatic Disorders hierarchy on the
Hierarchical SMQs
tab. An individual-level analysis for Liver Neoplasms, malignant, and unspecified would contain only those SMQs that fall within cell 20000011; the cumulative analysis would include SMQs from 20000011, and the sub-SMQs 20000208 and 20000209.
See
Graph Builder
documentation for more information.
|
•
|
A
Data Filter
.
|
This enables you to subset subjects based on demographic characteristics and other criteria. Refer to
Data Filter
for more information.
Summarizes hierarchical SMQs graphically and provides observed and cumulative
sample sizes
and percentages of SMQs.
The
Hierarchical SMQs
section contains the following elements:
The hierarchy plots illustrate the grouping relationship of several related
SMQs
. Tables below each hierarchy provide the counts and percents of subjects that experience the SMQs both individually and cumulatively.
|
•
|
Profile Subjects
: Select subjects and click
|
|
•
|
Demographic Counts
: Select subjects and click
|
|
•
|
Show SMQs
: Select SMQs from the plots and click
|
|
•
|
Show Events Leading to SMQs
: Select SMQs from the plots and click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click
|
|
•
|
Click the
arrow to reopen the completed report dialog used to generate this output.
|
|
•
|
Click the gray border to the left of the
Options
tab to open a dynamic report navigator that lists all of the reports in the review. Refer to
Report Navigator
for more information.
|
Include serious adverse events only
,
Event Type
,
Ignore available treatment emergent flags
,
Offset for End of Dosing
Additional Filter to Include Subjects
2
,
Merge supplemental domain
,
Include the following adverse events:
,
Filter to Include Adverse Events
,
Select the population to include in the analysis
Subject-specific filters must be created using the
Create Subject Filter
report prior to your analysis.
For more information about how to specify a filter using this option, see
The SAS WHERE Expression
.