Events Incidence Screen
This report screens all events from a domain by performing a Cochran-Mantel-Haenszel exact test on all 2 x 2 tables constructed from event (e.g. Medical History, Clinical Event) incidence and treatment arm. Output includes a volcano plot of risk difference when using default settings. However, plots of relative risks or odds ratios can be generated depending on the selected option for the x-Axis for Volcano Plot.
Note: This report should be considered as two different reports: DS Incidence Screen and MH Incidence Screen, depending on which domain is specified in the dialog.
Report Results Description
Running this report for Nicardipine using default settings for medical history (MH) generates the Report shown below.

Refer to the Adverse Events Incidence Screen output description, keeping in mind the following important report differences in contrast with the example:
| • | Output (including section names) reflects other event incidence corresponding to selected domain (e.g. Medical History, Clinical Events), rather than adverse events, incidence |
| • | There is no option to Perform Double FDR Adjustment |
| • | Term Level is allowed to equal Category (such as MHCAT or CECAT, depending on the domain). |
Refer to Confidence Intervals for information on how JMP Clinical calculates and uses confidence intervals.
Note: The following notes: Multiple treatment periods have been detected and displayed. and Pre-treatment has been assigned to period=0. are inserted at the top of the report when these events are detected in your data.
Report Options
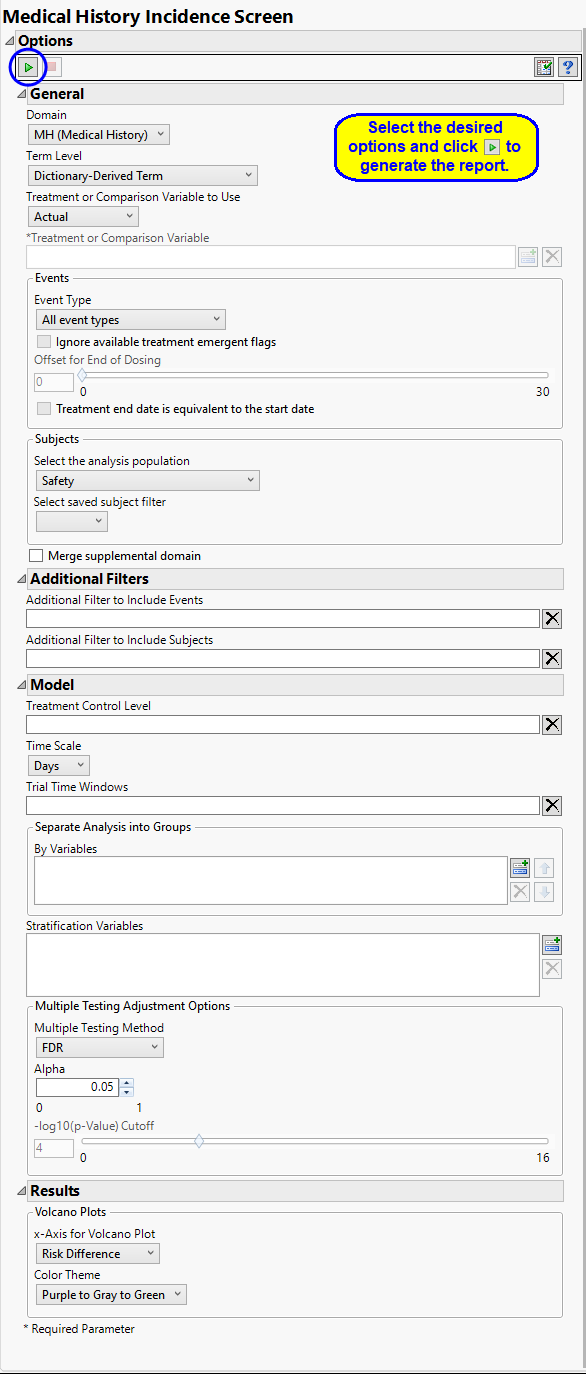
Domain
Use the Domain option to specify whether to examine results in Clinical Events (CE), Disposition (DS), Protocol Deviations (DV), Healthcare Encounters (HO) or Medical History (MH) events.
Term Levels
Term Levels are determined by the coding dictionary for the Event or Intervention domain of interest, typically these levels follow the MedDRA dictionary. You must indicate how each adverse event is named and the level at which the event is considered. For example, selecting Reported Term as the Term Level reports the event specified by the actual event term as reported in the AE domain.
Treatment or Comparison Variable:
The primary goal of clinical trials is to distinguish treatment effects when reporting and analyzing trial results. Treatments are defined by specific values in the treatment or comparison variables of the CDISC models. These variables are specified in this report using the Treatment or Comparison Variable to Use andTreatment or Comparison Variable options.
Distributions of the specified treatment or comparison variables are shown in the output.
Available variables include Planned, which is selected when the treatments patients received exactly match what was planned and Actual, which is selected when treatment deviates from what was planned.
You can also specify a variable other than the ARM or TRTxxP (planned treatment) or ACTARM or TRTxxA (actual treatment) from the CDISC models as a surrogate variable to serve as a comparator. Finally you can select None to plot the data without segregating it by a treatment variable.
See Treatment or Comparison Variable to Use, Treatment or Comparison Variable for more information.
Events to Consider
Analysis can consider all events or only those that emerge at specific times before, during, or after the trial period. For example, selecting On treatment events as the Event Type includes only those events that occur on or after the first dose of study drug and at or before the last dose of drug (+ the offset for end of dosing).
If you choose to Ignore available treatment emergent flags, the analysis includes all adverse events that occur on or after day 1 of the study.
By default, post-treatment monitoring begins after the patient receives the last treatment. However, you might want to specify an Offset for End of Dosing, increasing the time between the end of dosing and post-treatment monitoring for treatments having an extended half-life.
Check the Treatment end date is equivalent to the start date if the treatment end date (EXTENDTC) is missing from the data. In this case, it is assumed that all treatments were given on the same day and that the treatment start date can be used instead.
Filtering the Data:
Filters enable you to restrict the analysis to a specific subset of subjects and/or adverse events, based on values within variables. You can also filter based on population flags (Safety is selected by default) within the study data.
If there is a supplemental domain (SUPPDS or SUPPMH) associated with your study, you can opt to merge the non-standard data contained therein into your data.
See Select the analysis population, Select saved subject filter1, Merge supplemental domain, Additional Filter to Include Events, and Additional Filter to Include Subjects
Model
The Treatment Control Level is specified as either “Placebo” or “Pbo”, depending on the value found in your data, by default. However, if your control is defined differently you can use the text box to specify the control level is identified in your study.
You can opt to assess interventions across the entire study (specified by default). Alternatively, you can use the Trial Time Windows option to limit it to selected time points or intervals. By default, time is measured in days. However, you can change the Time Scale to measure time in weeks. This option is useful for assessing report graphics for exceptionally long studies.
You can also subdivide the subjects and run analyses for distinct groups by specifying one or more By Variables.
Stratification Variables segment the data into meaningful clusters and statistical tests such as the CMH exact test account for them. Such stratification is usually recommended when strata exist in your study, for example, by site.
Multiple Testing Adjustment Options
Multiple testing adjustments enable you to control both the proportion of all truly null hypothesis tests that are claimed to be significant (false positive rate) and the proportion of hypothesis tests claimed significant that are truly null (false discovery rate) for conclusions drawn across a collection of statistical tests.
The False Discovery Rate test is selected by default. You can use the Multiple Testing Method option to select alternative test protocols.
The Alpha option is used to specify the significance level by which to judge the validity of the summary statistics generated by this report. The meaning of alpha depends on the adjustment method that you select. Alpha can be set to any number between 0 and 1, but is most typically set at 0.001, 0.01, 0.05, or 0.10. The higher the alpha, the higher the error rate but also higher the power for detecting significant differences. You will need to decide on the best trade-off for your experiment.
Note that instead of performing multiple testing adjustments of the p-values, you can opt to simply specify a cutoff value for -log10(p-values) in order to select significant hypothesis tests. Using unadjusted p-values with a cutoff has the benefit of more expansive volcano plots, whereas adjusted p-values tend to squish points along the y-axis.Refer to -log10(p-Value) Cutoff for more information. Note: This option is available only when no multiple testing method is specified.
Output
Use the x-Axis for Volcano Plot option to specify the parameter to be plotted on the x-axis of your volcano plots. The Color Theme option enables you to specify the color scheme of the report graphics.