JMP Clinical Main Window
When you open JMP Clinical, the first window you see is the JMP Clinical Main window:
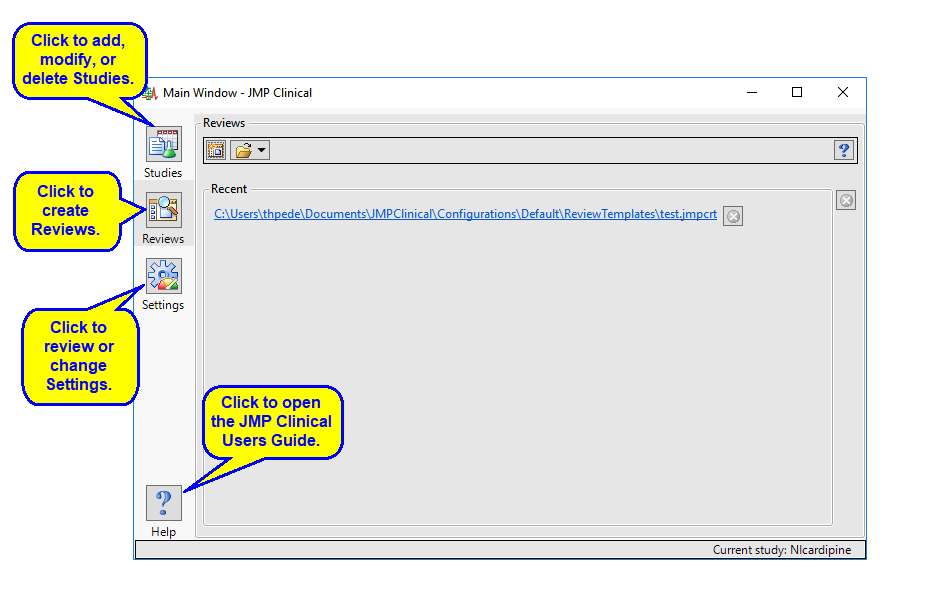
There are four buttons aligned on the left side of the JMP Clinical Main Window: Studies ( ), Reviews (
), Reviews ( ), Settings(
), Settings( ), and Help(
), and Help( ). The Reviews tab is selected by default.
). The Reviews tab is selected by default.
Click on the following links for more information:
The Studies Tab
Adding and Manipulating Studies
The Reviews Tab
The Settings Tab
A Note about User Roles
Before we can discuss specifics about each of the tabs on the JMP Clinical main window, we need to introduce the concept of the User Role in performing JMP Clinical analyses. JMP Clinical is designed as a multi-functional tool for setting up a study with the associated data, running a variety of reports and analyses, and reviewing the results.
By default a user can perform all of these functions and have access to all of the described functions and tabs. However, depending on the needs of your organization, these functions can be divided among different users, each carrying out a specific role. To facilitate these roles, JMP Clinical can be configured to provide individual users with the functional capabilities needed to carry out their assigned roles. As a result, when specific user roles are assigned, the JMP Clinical main window changes to show users only those functions specific to their assigned user role.
Refer to The JMP Clinical Main Window Changes Depending on the Assigned User Role. for more information.
Additional Information
Refer to Available Reports for links to information for each of the JMP Clinical reports.
Refer to Introduction for information about:
| • | System Requirements, Text Conventions, How is JMP Clinical affected by localization-specification?, Adverse Events Narratives, and The Example Data. |
| • | Frequently Asked Questions |
| • | System Operations and how JMP Clinical interacts with the clinical and nonclinical study data that is added to a JMP Clinical Study |
Refer to the Appendixes for a Glossary, Trouble Shooting guide, and other useful information.