Site Details for Risk Based Monitoring Report
Users can set up a file that contains additional information about the sites compared to what is captured through the eCRFs. This file can have the following data:
-
Geographic parameters of the sites:
-
Country
-
City
-
State / Province
-
Zip Code
-
Latitude
-
Longitude
-
-
Site Active Date - the date when the site became active. This is used to calculate risk indicators concerning enrollment rates
-
Site Category - sites can be grouped, e.g.: Novice, Experienced, and Veteran
-
Monitor - name or ID of the monitor
-
Vendor - name or ID of the vendor
Note: The Site Details data file is optional. The Risk Based Monitoring report can be run without it. However, certain options within this report are not available unless this data table is present in the correct location. If users want to run analysis at Monitor or Vendor level and if they want risk indicators concerning enrollment rates, then this file needs to be set up.
The Site Details data file is a JMP table and must be named rbm_site. It must be placed in the C:\Users\username\JMPClinical18\Configurations\Default\Notes\Study Name directory on Windows and in ** on Macs, where username corresponds to the user's user identifier on the computer and Study Name corresponds exactly to the name of the study. If the Study Name folder does not exist in the appropriate file path listed above, it must be created.
Example
A Site Details data table for the Nicardipine example is included with JMP Clinical and it is called NicardipineRBM_en.jmp for English locale. If locale is Chinese, then the file is Nicardipine_zh.jmp and if it is Japanese, then the file is NicardipineRBM_zh.jmp. This file can be found in the Notes directory of the Nicardipine study, if Nicardipine is registered in JMP Clinical (C:\Users\username\JMPClinical18\Configurations\Default\Notes\Nicardipine on Windows and in ** on Macs) or in the installation folder (C:\ProgramData\JMP\JMPClinical\18\Clinical\Sample Data\Nicardipine on Windows and in ** on Macs) .
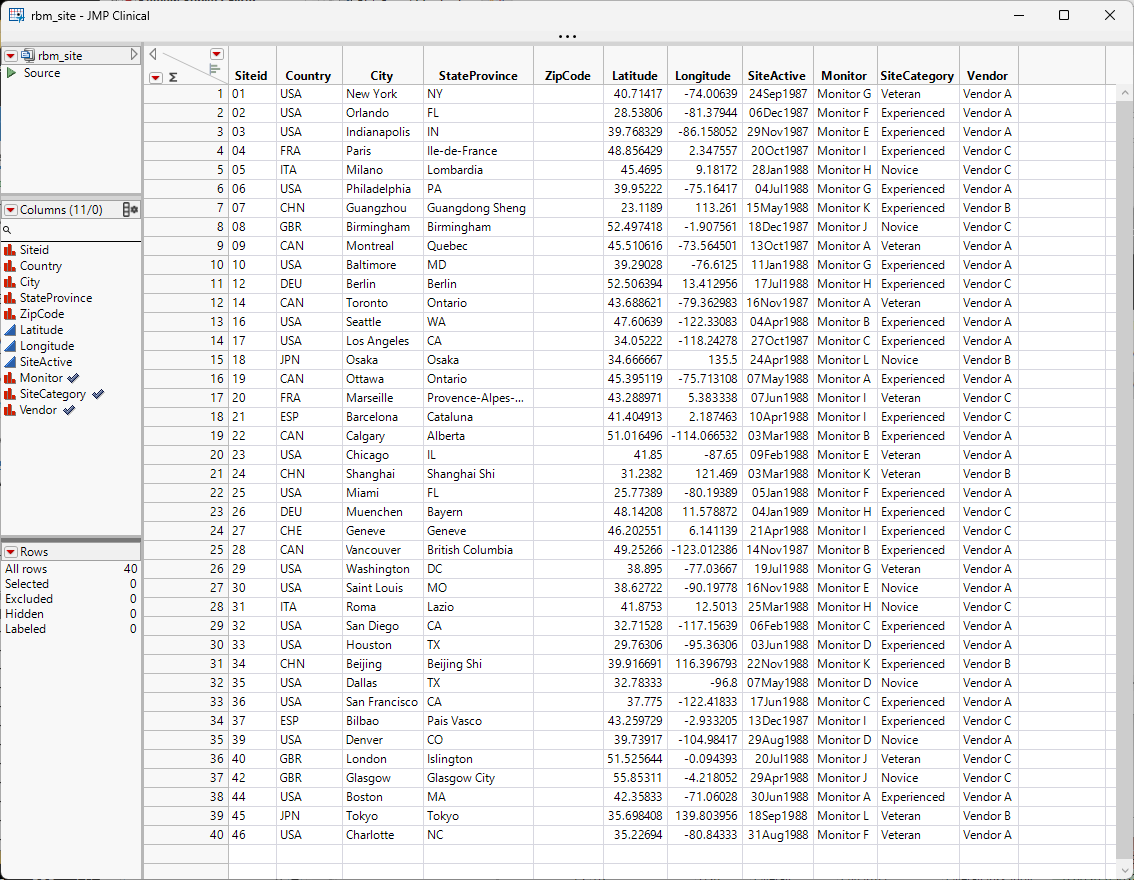
Note especially the three highlighted columns; these correspond to options on the RBM report.
Generating the Study Risk Data Set
Users must generate the Site Details data table and enter site-specific descriptive information. This can be done either by opening a new JMP table and manually entering the data or modify an existing Site Details data table. In both cases:
| • | The new data table must be named rbm_site. |
| • | Only the Siteid column is required, all other columns are optional. |
| • | The names of the included columns must exactly match the names in the example shown above. |
| • | The new data table must be saved to the appropriate folder (C:\Users\username\JMPClinical18\Configurations\Default\Notes\Study Name directory on Windows and in ** on Macs). |
| • | The columns of this file should have the following parameters: |
| Column Name | SAS Name | SAS Label for English locale | Data Type | Modeling Type | Format |
|---|---|---|---|---|---|
| SITEID | SITEID |
Study Site Identifier |
Character | Nominal | |
| COUNTRY | COUNTRY | Country | Character | Nominal | |
| CITY | CITY | City | Character | Nominal | |
| STATEPROVENCE | STATEPROVENCE | State or Province | Character | Nominal | |
| ZIPCODE | ZIPCODE | Five Digit Zip Code | Character | Nominal | |
| LATITUDE | LATITUDE | Latitude | Numeric | Continuous | Format: Best, Width: 12 |
| LONGITUDE | LONGITUDE | Longitude | Numeric | Continuous | Format: Best, Width: 12 |
| SITEACTIVE | SITEACTIVE | Site Active Date | Numeric | Continuous | Format: ddMonyyyy, Width: 10 |
| MONITOR | MONITOR | Monitor | Character | Nominal | |
| SITECATEGORY | SITECATEGORY | Site Category | Character | Nominal | |
| VENDOR | VENDOR | Vendor | Character | Nominal |
Note: In order to add SAS Name and SAS Label column properties, you will need to right click on the column, click on Column Properties and select Other from the drop-down. Enter the property name, which is SAS Name or SAS Label, then enter a value for this property.