Site Details for Risk Based Monitoring Report
Users can set up a file that contains additional information about the sites compared to what is captured through the eCRFs. This file can have the following data:
-
Geographic parameters of the sites:
-
Country
-
City
-
State / Province
-
Zip Code
-
Latitude
-
Longitude
-
-
Site Active Date - the date when the site became active. This is used to calculate risk indicators concerning enrollment rates
-
Site Category - sites can be grouped, e.g.: Novice, Experienced, and Veteran
-
Monitor - name or ID of the monitor
-
Vendor - name or ID of the vendor
Note: The Site Details data file is optional. The Risk Based Monitoring report can be run without it. However, certain options within this report are not available unless this data table is present. If users want to run analysis at Monitor or Vendor level and if they want risk indicators concerning enrollment rates, then this file needs to be set up.
Example
A Site Details data table for the Nicardipine example is included with JMP Clinical and it is called NicardipineRBM_en.jmp for English locale. If locale is Chinese, then the file is Nicardipine_zh.jmp and if it is Japanese, then the file is NicardipineRBM_zh.jmp. This file can be found in the Notes directory of the Nicardipine study, if Nicardipine is registered in JMP Clinical (C:\Users\username\JMPClinical18\Configurations\Default\Notes\Nicardipine on Windows and in ** on Macs) or in the installation folder (C:\ProgramData\JMP\JMPClinical\18\Clinical\Sample Data\Nicardipine on Windows and in ** on Macs) .
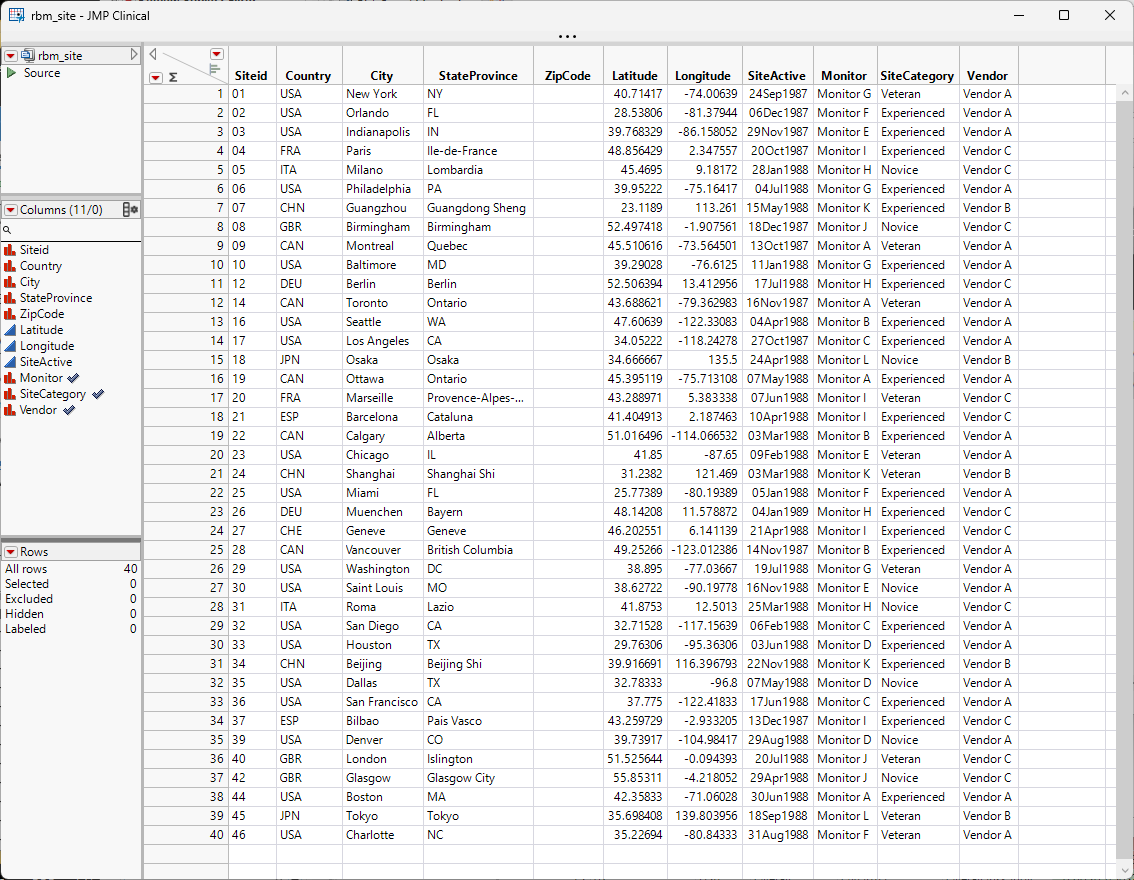
Note especially the three highlighted columns; these correspond to options on the RBM report.
Generating the Study Risk Data Set
Users must generate the Site Details data table and enter site-specific descriptive information. This is done using the Update Site Details for Risk Based Monitoring action button on the Studies tab.