Add Study...
This report adds a study (see Studies) from either local folders or a SAS Drug Development (SDD) server that contain CDISC-formatted data. You must add a study before you can perform the analysis. This report can also import SAS transport files for you.
The following table summarizes the minimum requirements of new studies.
|
You must specify... |
...while abiding by these restrictions: |
||||||||||||||||||
|
|||||||||||||||||||
| Type of Study |
|
||||||||||||||||||
|
the location of either the SDTM or SEND Folder or the ADaM Folder |
|
The study and associated metadata are added to your database.
To Add a Study
| 8 | Click  to open the dialog: to open the dialog: |
Note: If your JMP Clinical software has been configured for SAS Drug Development, clicking  expands a drop-down menu. Select From Folders from the drop-down menu to open the Add Study dialog.
expands a drop-down menu. Select From Folders from the drop-down menu to open the Add Study dialog.
| 8 | Specify the desired options and click . |
The following options are available. Links to specific documentation for each of the options are provided in the following table:
|
Options |
Information |
||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
Selecting this option enables future snapshot comparisons of study data collected and recorded at different intervals during the study period. |
|||||||||||||||||||||||||
Note: Relationship data sets cannot be excluded and, therefore, are not listed. |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
Make this the current study |
|
Note: When adding a clinical study and assigning both ADAM and SDTM domains, if you do not explicitly specify which SDTM and ADAM domains to include, the ADAM equivalent of each domain is added and the corresponding SDTM data set excluded. For example, if both ADAE and AE are present, then ADAE takes precedence and is included, whereas AE is not included.
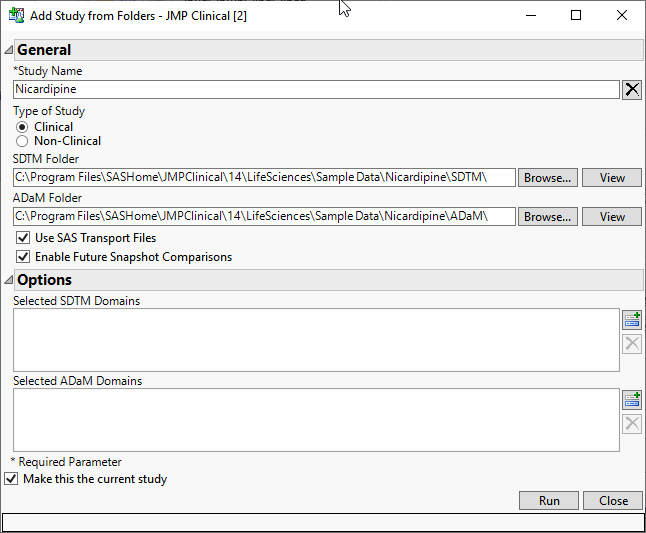
Example: Adding the Nicardipine Study
JMP Clinical ships with the data from a Nicardipine study. The data files are located in the Sample Data in your Installation directory (typically C:\Program Files\SASHome\JMPClinical\14\LifeSciences\Sample Data\Nicardipine). In this example, we add a clinical study we call Nic1.
| 8 | Click  to open the Add Study dialog. to open the Add Study dialog. |
| 8 | Specify the name of the study. |
| 8 | Click the Clinical radio button to specify the type of study. |
| 8 | Specify the file paths to the SDTM and ADaM folders either by typing the path directly or clicking |
| 8 | Check the boxes to enable use of SAS transport files and future snapshot comparisons. |
The completed dialog appears as follows:
| 8 | Click to add the study. |
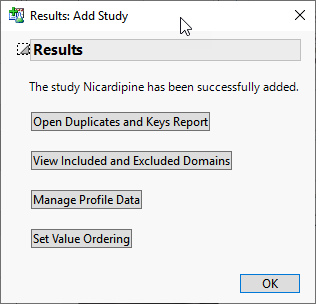
When a study has been successfully added, the Results: Add Study window opens:
The following options are available. Links to specific documentation for each of the options are provided in the following table:
|
Options |
Information |
|||
|
|
|
|||
|
|
|
|||
|
|
|
|||
|
|
|