Correlated Findings
This report calculates pairwise correlations between tests within each findings domain and identifies unusual results at specific study sites.
Report Results Description
Running Correlated Findings for Nicardipine using default settings generates the report shown below.
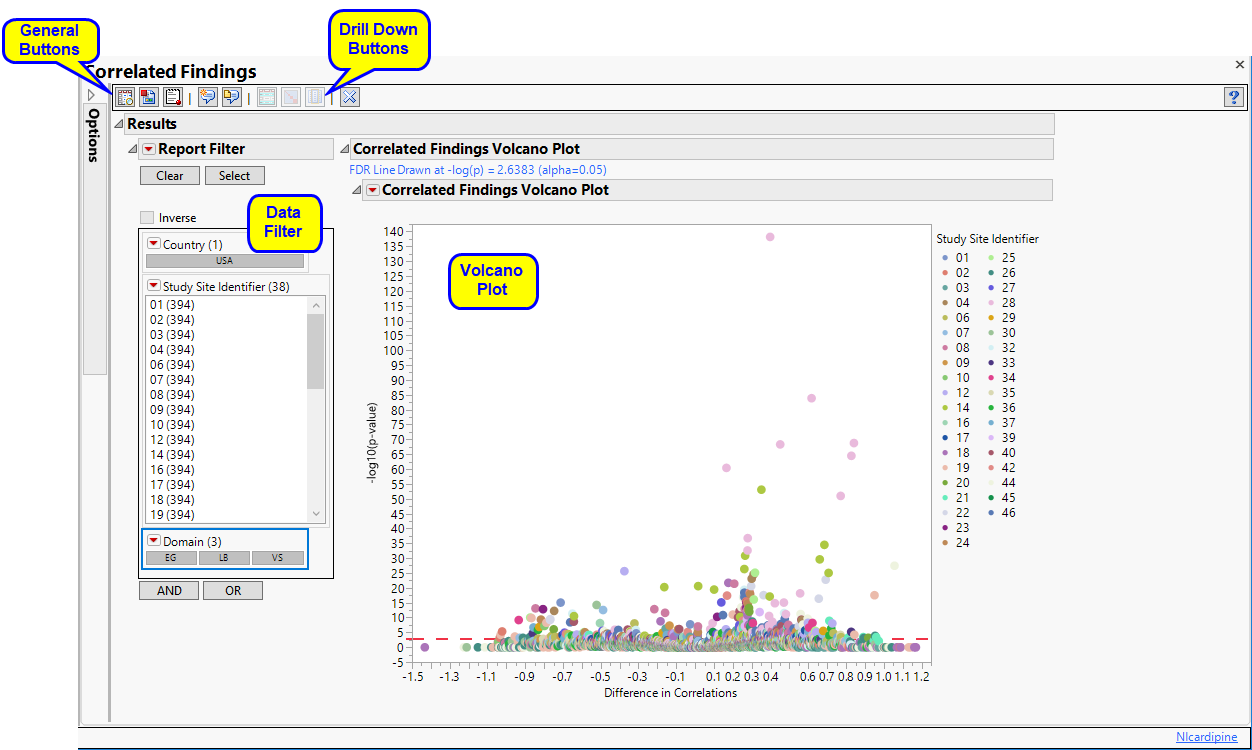
The Report contains the following elements:
Correlated Findings Volcano Plot
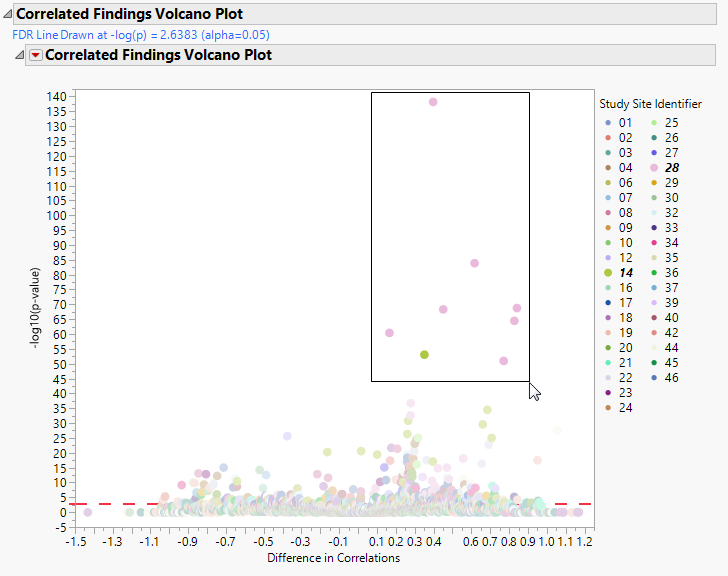
This plot show points representing the correlations between findings. Points close to zero show little or no correlation. Correlations increase the further (either positively or negatively) the further they are from zero. Selected points, in the figure below, are positively correlated.
Data Filter
This enables you to subset subjects based on demographic characteristics and study site. Refer to Data Filter for more information.
Action Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report. Use your mouse to select one or more sites of interest before selecting a drill down option, as shown below:
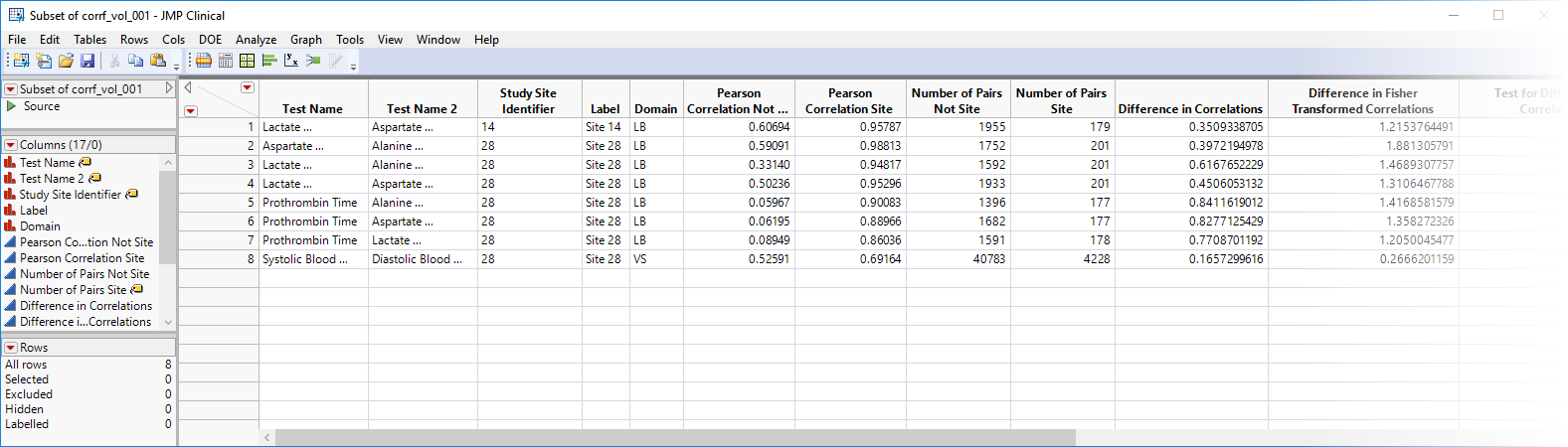
| • | Show Sites: Shows the rows of the data table for the selected points from the volcano plot. Clicking  opens the following table: opens the following table: |
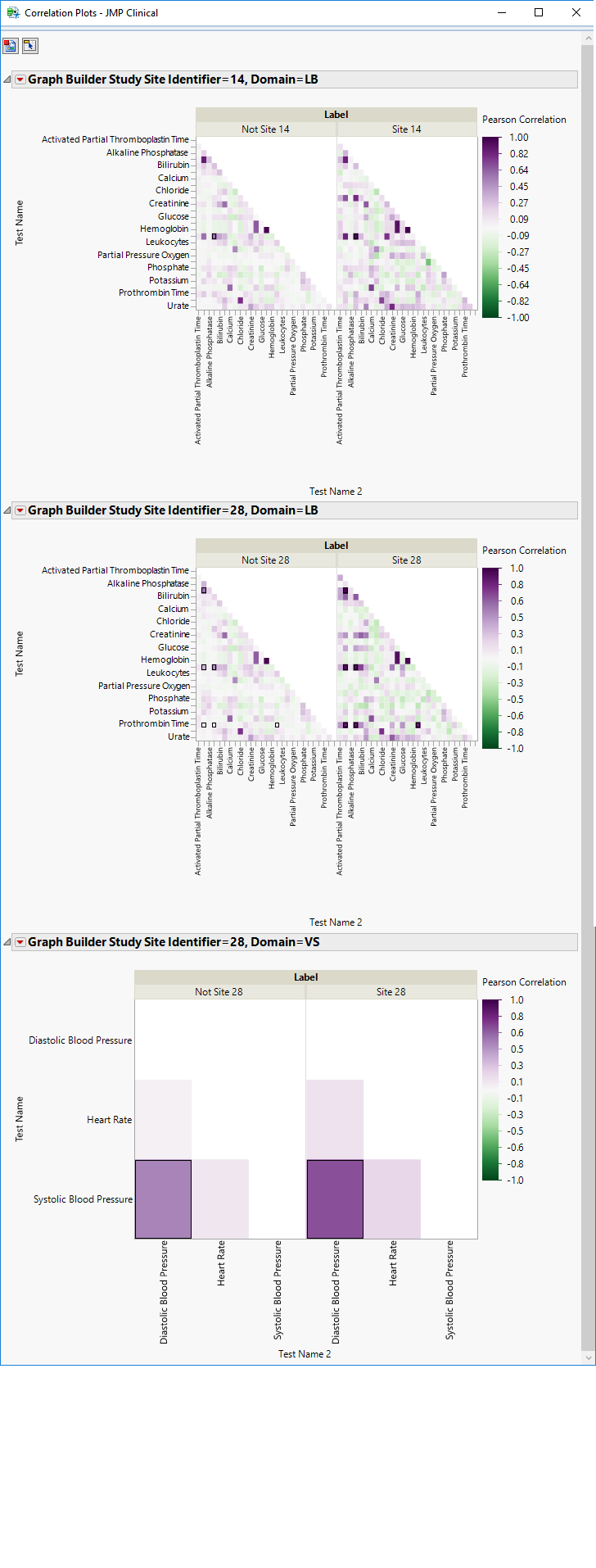
| • | Show Correlation Plot: Click  to display correlation plots for the selected points. to display correlation plots for the selected points. |
| • | Show Findings: Click  to display all a table of the selected correlated findings. to display all a table of the selected correlated findings. |
General
| • | Click  to view the associated data tables. Refer to View Data for more information. to view the associated data tables. Refer to View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click  to open and view the Subject Explorer/Review Subject Filter. to open and view the Subject Explorer/Review Subject Filter. |
| • | Click  to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. |
| • | Click the arrow to reopen the completed report dialog used to generate this output. |
| • | Click the gray border to the left of the Options tab to open a dynamic report navigator that lists all of the reports in the review. Refer to Report Navigator for more information. |
Methodology
Fisher's transformation (Fisher, 19211, 19702) of the correlation coefficient is calculated for all pairs of tests by CDISC findings domain, with each site (the suspect site, indexed with s) compared to all other sites taken together as a reference (indexed as o). Corresponding p-values are calculated according to a normal approximation.

FDR p-values are calculated and the reference line is determined as described in How does JMP Clinical calculate the False Discovery Rate (FDR)?.
Report Options
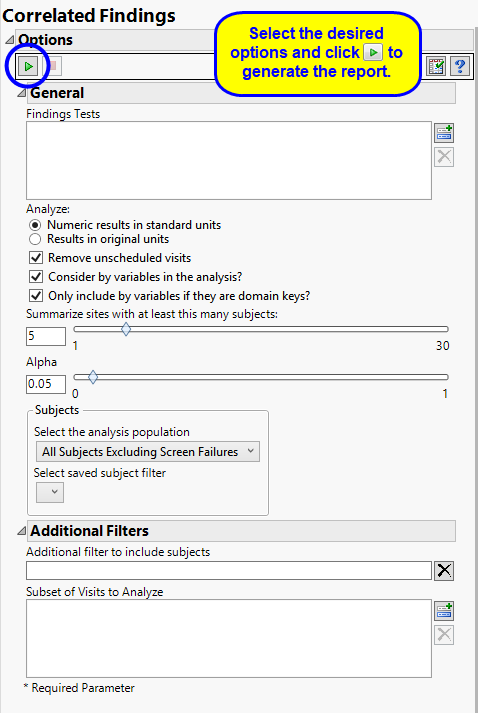
By default the report is set to analyze all tests from all findings domains. You can opt however, to restrict the search to specific Findings Tests.
You can specify whether to search all results in original units when you Analyze: the data or restrict the search to either character results (in standard format) or numeric results (in standard units).
You might or might not want to include unscheduled visits when you are analyzing findings by visit. Check the Remove unscheduled visits to exclude unscheduled visits.
You can opt to Consider BY variables in the analysis. This option, which assumes that BY variables (left vs. right arm for collecting blood pressure data, for example) are included in the experimental design, is selected by default. You can uncheck this option to ignore BY variables.
Use the Only include BY variables if they are domain keys option to subset the available variables to only include those variables that are domain keys. If the option is unchecked, the report uses the cross-classification of xxCAT, xxSCAT, xxLOC, xxMETHOD, xxPOS, xxSPEC, and xxTPT for creating by groups for all variables that are available (as it had in the past).
The Summarize sites with at least this many subjects: option enables you to set a minimal threshold for the sites to be analyzed. Only those sites which exceed the specified number of subjects are included. This feature is useful because it enables you to exclude smaller sites, where small differences due to random events are more likely to appear more significant than they truly are. In larger sites, observed differences from expected attendance due to random events are more likely to be significant because any deviations due to random events are less likely to be observed.
The Alpha option is used to specify the significance level by which to judge the validity of the correlations generated by this report. By definition, alpha represents the probability that you will reject the null hypothesis when the null is, in fact, true. Alpha can be set to any number between 0 and 1, but is most typically set at 0.01, 0.05, or 0.10. The higher the alpha, the lower your confidence that the results you observe are correct.
Filtering the Data:
Filters enable you to restrict the analysis to a specific subset of subjects and/or adverse events, based on values within variables. You can also filter based on population flags (Safety is selected by default) within the study data.
See Select the analysis population, Select saved subject filter3, and Additional Filter to Include Subjects
The Subset of Visits to Analyze options enables you to restrict to a specific subset of visits your search tests with similar and questionable results.