Events Multiple Occurrences Distribution
**
This report enables you to compare distributions of events across treatment arms for subgroups defined by demographics such as age, sex and race. It computes counts based on the overall counts of events, including multiple occurrences per subject.The number of events is reported per term. occurrences per subject.
Note: This report should be considered as two different reports: Disposition Multiple Occurrences Distribution and Medical History Multiple Occurrences Distribution, depending on which domain is specified in the dialog.
Note: Refer to Distribution Reports for a description of the general analysis performed by the JMP Clinical distribution reports.
Report Results Description
Running this report for Nicardipine using default settings for medical history (MH) generates the Report shown below.
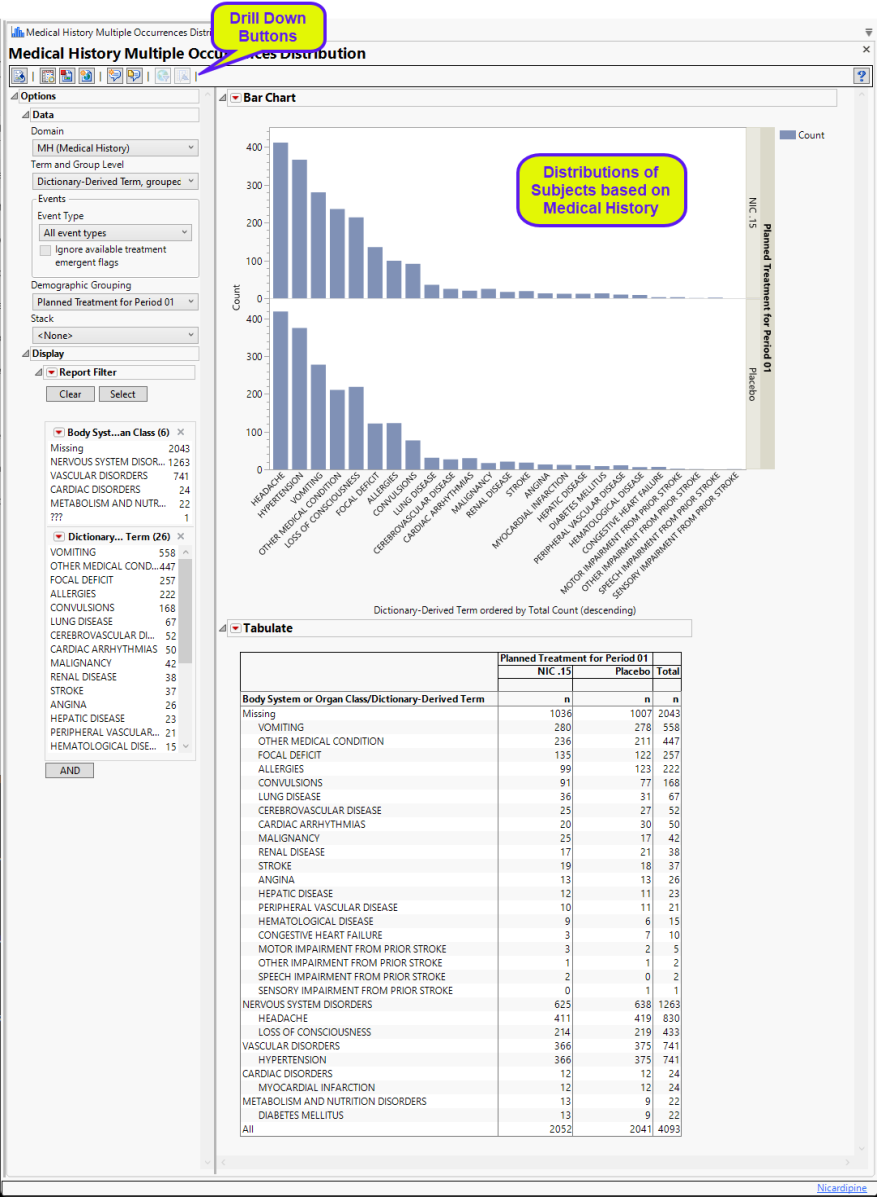
Refer to the Adverse Events Multiple Occurrences Distribution output description, including all of the Report elements and areas where Events Multiple Occurrences Distribution differs from Adverse Events Multiple Occurrences Distribution.
Note: The following notes: Multiple treatment periods have been detected and displayed. and Pre-treatment has been assigned to period=0. are inserted at the top of the report when these events are detected in your data.
Options
Data
Use the drop-downs on this panel to customize your analysis.
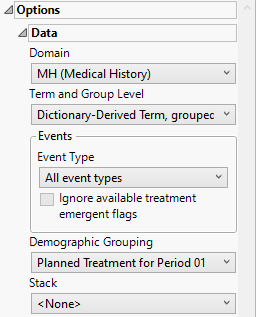
Domain
Use the Domain option to specify whether to plot the distribution of either disposition (DS) or medical history (MH) events.
Term and Group Level
Term and Group Levels are determined by the coding dictionary for the Event or Intervention domain of interest, typically these levels follow the MedDRA dictionary. Use this widget to indicate how each adverse event is named and the level at which the event is considered. For example, selecting Reported Term for the Adverse Event, grouped by Body System or Organ Class as theTerm and Group Level reports the event specified by the actual event term as reported in the AE domain on the affected organ or body system.
The term level will determine which term is displayed on the x-axis of the bar chart. Both the term and group level will be shown in the table with term level nested within group level.
Event Type
Analysis can consider all events or only those that emerge at specific times before, during, or after the trial period. For example, selecting On treatment events as the Event Type includes only those events that occur on or after the first dose of study drug and at or before the last dose of drug (+ the offset for end of dosing).
Ignore available treatment emergent flags
If you choose to Ignore available treatment emergent flags, the analysis includes all adverse events that occur on or after day 1 of the study when the event type is Treatment emergent events.
Demographic Grouping and Stack
Results can be viewed as a whole or they can either be split out by Demographic Grouping or Stacked to show different levels within each event. Demographic Grouping will change the variable displayed on the y-axis of the bar chart, as well as change the variable used across the top of the table to split the columns. Stack impacts both the bar chart by stacking the bars according to the levels of the chosen variable with corresponding effects in the table. For example, if severity is chosen, the bars will be stacked by mild, moderate, and severe events
Display
Report Data Filters
These filters enable you to subset and view subjects based on demographic characteristics and other criteria. Refer to Data Filter for more information.
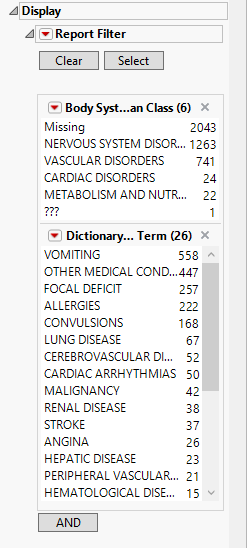
Note: Filter specifications are reapplied when any widget options are changed.
General and Drill Down Buttons
Action buttons, provide you with an easy way to drill down into your data. The following action buttons are generated by this report:
| • | Click  to rerun the report using default settings. to rerun the report using default settings. |
| • | Click  to view the associated data tables. Refer to Show Tables/View Data for more information. to view the associated data tables. Refer to Show Tables/View Data for more information. |
| • | Click  to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. to generate a standardized pdf- or rtf-formatted report containing the plots and charts of selected sections. |
| • | Click  to generate a JMP Live report. Refer to Create Live Report for more information. to generate a JMP Live report. Refer to Create Live Report for more information. |
| • | Click  to take notes, and store them in a central location. Refer to Add Notes for more information. to take notes, and store them in a central location. Refer to Add Notes for more information. |
| • | Click  to read user-generated notes. Refer to View Notes for more information. to read user-generated notes. Refer to View Notes for more information. |
| • | Click  to open and view the Review Subject Filter. to open and view the Review Subject Filter. |
| • | Click  to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. to specify Derived Population Flags that enable you to divided the subject population into two distinct groups based on whether they meet very specific criteria. |
Default Settings
Refer to Set Study Preferences for default Subject Level settings.
Methodology
No testing is performed. Analysis is restricted to tabulating countsof either medical history or disposition events.