How Supplemental Data Sets are Used by JMP Clinical.
Prior to version 6.0, analyses using the Risk Based Monitoring (RBM) report were based entirely on one or more domains of the study database and data supplied at the site level. Data was entered manually using Update Study Risk Data Set. However, this protocol is inadequate for the study team that wishes to assess data quality for data captured outside of the study database, for endpoints that are captured at either the patient or site level, with considerations for when the data was captured. This includes data from database management systems (queries, CRF entry), randomization systems, protocol deviations or eligibility violations identified through statistical programming, or any deviations identified by clinical monitors while on site. RBM analyses can accommodate a supplemental data set. This enhancement enables clinical trial sponsors to specify a data set of additional endpoints that can be included in the RBM report.
The supplemental data set is not considered part of the study folders that can be updated periodically in the form of snapshots, largely because of the variable timing these other data might become available. However, the supplemental data can include complex calculations of the current, more-recent or any past study database for inclusion into the RBM analysis. For example, through the RBM dialog users can subset to particular adverse events or health care encounters of interest for analysis. For example, if users were interested solely in adverse events that occurred during health care encounters, the statistical team can develop programming to identify these adverse event records and supply them using the supplemental data set for analysis.
Refer to Risk-Based Monitoring Supplemental Data Table for more information, along with specific examples, on the Supplemental standard.
Specifying a Supplemental Data Set
A supplemental data can be specified through the Risk-Based Monitoring report(circled below).
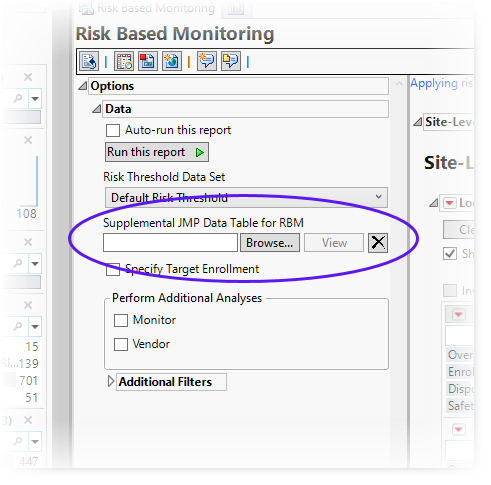
By default, JMP Clinical looks first in the RBMSupplemental subfolder found in the user folder (typically located in the C:\Users\username\OneDrive - SAS\Documents\JMPClinical18\Configurations\Default\RBMSupplemental\rb_en.jmp directory), although supplemental data sets can be stored anywhere.

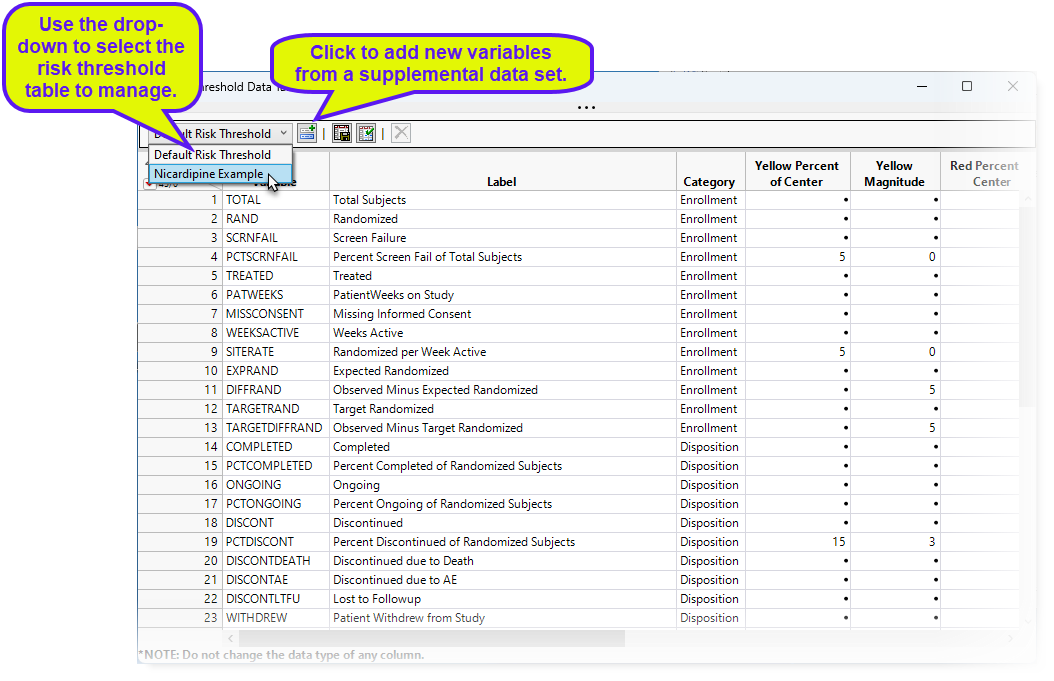
The Nicardipine sample data included with JMP Clinical, has an example supplemental data set (rb_en.jmp, English version).
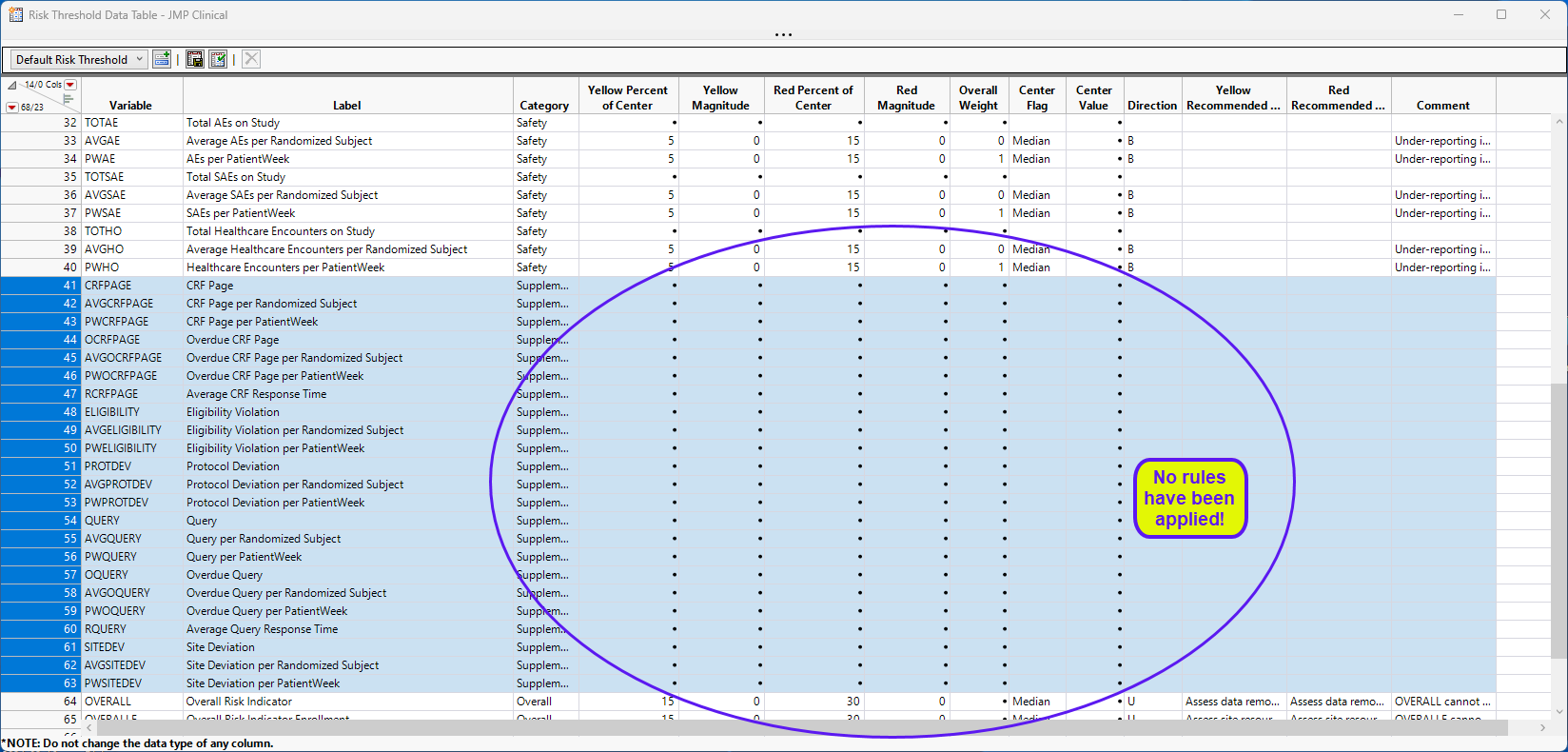
This risk threshold data set includes rows for the variables included in the supplemental data set, as well as:
| • | Risk indicators normalized by the number of patients |
| • | Risk indicators normalized by the number of patient weeks |
| • | Additional indicators for the number of incomplete CRF pages and queries, as well as average times for completion based on the usage of special variable codes as documented in the supplemental standard above. |
Running this analysis will include these variables in the output and consider the variables for any overall indicators that they happen to be a part of.
Note: The following changes have made to JMP Clinical 6,1
| • | No endpoint data can be supplied through Update Study Risk Data Set. All subject and site level variables are now included using the supplemental data set. |
| • | Variables entered manually are now referred to as supplemental variables in the Risk-Based Monitoring output. |
Updating the Study Risk Data Set With Supplemental Data
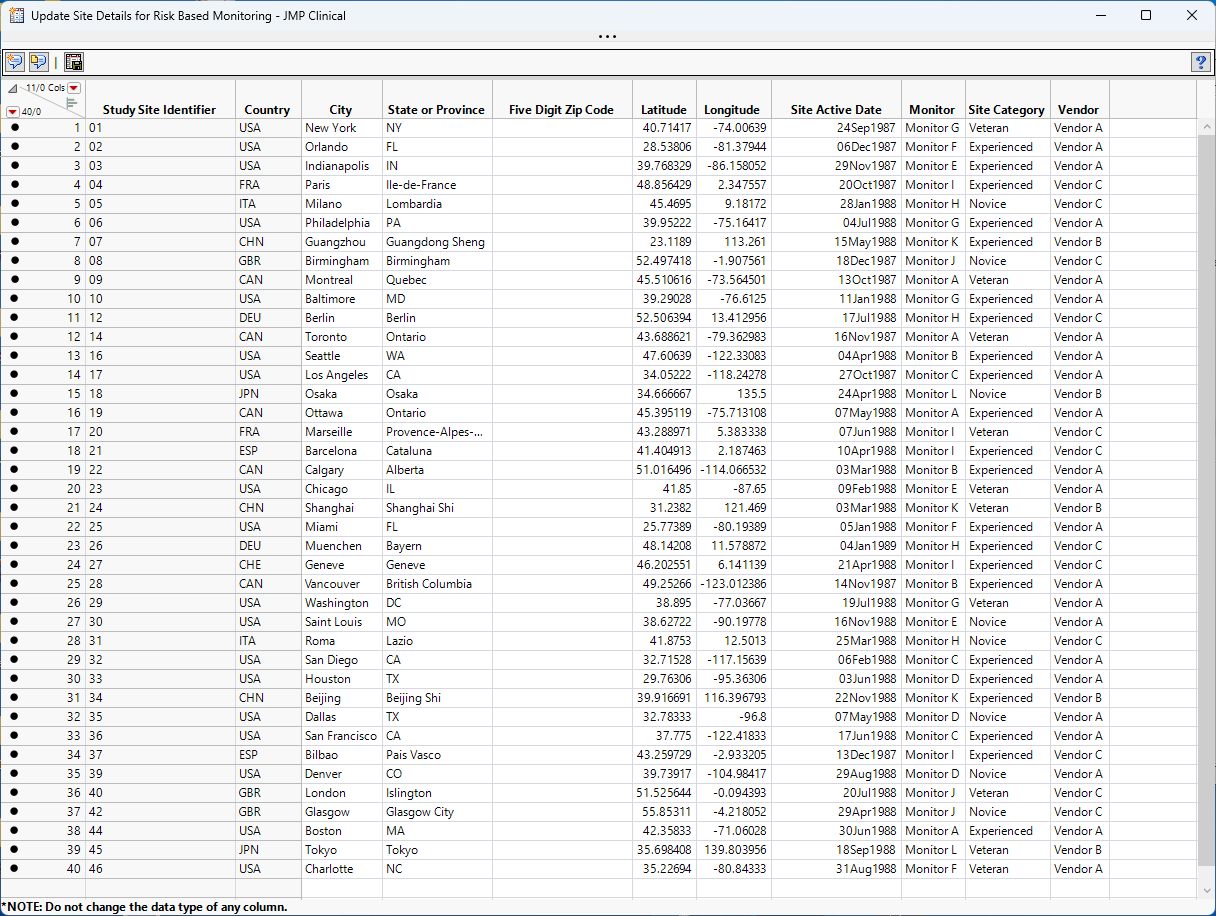
The Update Site Details for Risk Based Monitoring option is accessed using an action button on the Studies tab of the JMP Clinical Main . (See below.)
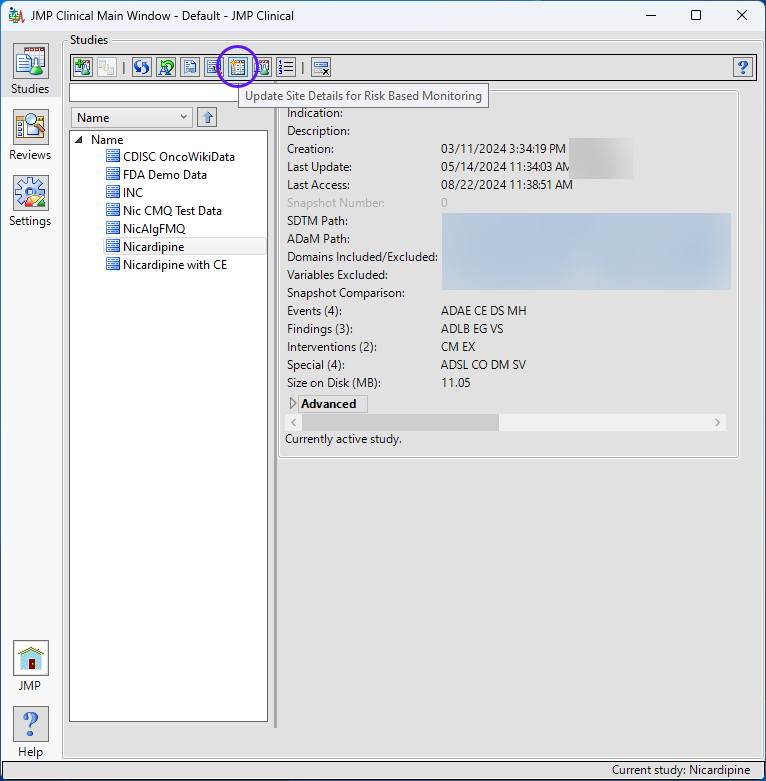
Clicking this action button opens the Study Risk data set (shown below). Note that only site level classifications are supplied.
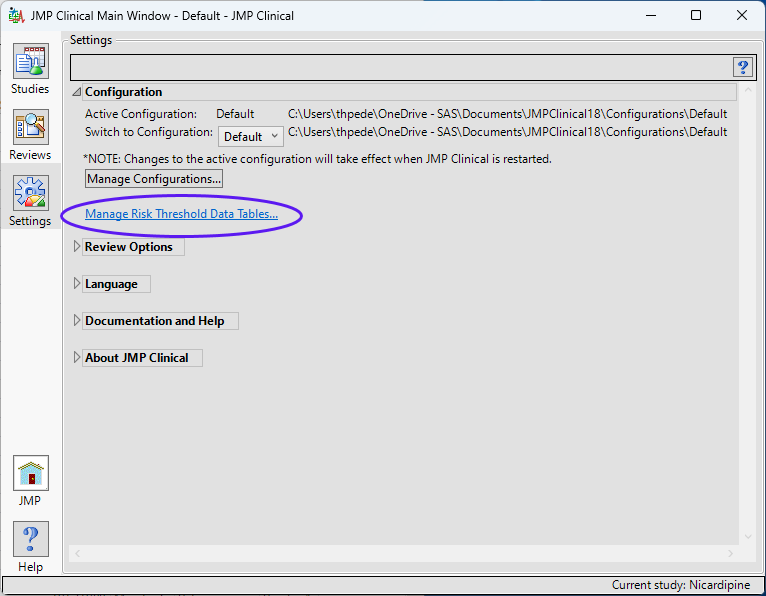
To add supplemental indicators, you first need to manage risk threshold data sets (which enables you to delete, update, or add new variables to a base risk threshold data set). The Risk Threshold data set is accessed from a link on the Settings tab of the JMP Clinical Main (below).
Clicking Manage Risk Threshold Data Tables... opens the default risk threshold data set (shown below).
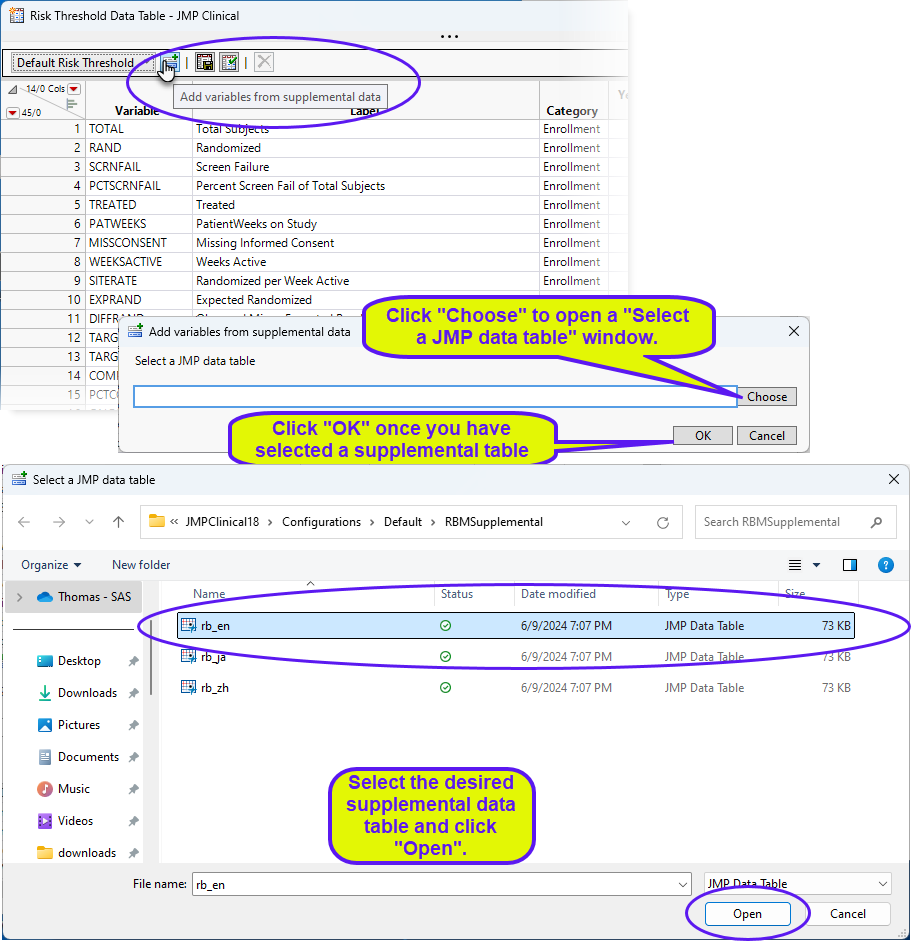
To add new variables from a supplemental data set, click  . An Add variables from supplemental data opens. Click to open a Select a SAS data set showing the contents of the supplemental data set folder. Select the desired supplemental data set and click . Return to the Add variables from supplemental data and click .
. An Add variables from supplemental data opens. Click to open a Select a SAS data set showing the contents of the supplemental data set folder. Select the desired supplemental data set and click . Return to the Add variables from supplemental data and click .
This report adds a number of additional variables (Supplemental, because this is the defined Category in the rb_en.sas7bdat data set). This data set can now have rules applied to the variables and can be saved with a new name to be used by theRisk Based Monitoring report. Note that the only difference between the data set shown below and the Nicardipine Example risk threshold data set is that there are no rules defined in the data set shown below. The data set as defined below enables an analysis of the supplemental data, but without any coloring of the risk indicators according to any rules.